Drug Catalog - Product Detail
OSELTAMIVIR ORAL SOL 6MG/ML 60ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 47781-0384-26 | ALVOGEN | 60 | 6MG/ML | SUSPENSION |
PACKAGE FILES


Generic Name
OSELTAMIVIR PHOSPHATE
Substance Name
OSELTAMIVIR PHOSPHATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA208823
Description
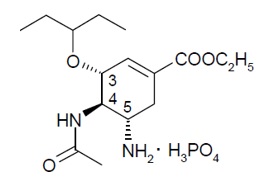
11 DESCRIPTION Oseltamivir phosphate, USP, an influenza neuraminidase inhibitor (NAI), is available as a powder for oral suspension, which when constituted with water as directed contains 6 mg per mL oseltamivir base. In addition to the active ingredient, the powder for oral suspension contains colloidal silicon dioxide, monosodium citrate, saccharin sodium, sodium benzoate, sorbitol, titanium dioxide, tutti-frutti flavoring and xanthan gum. Oseltamivir phosphate, USP is a white to off-white powder with the chemical name (3R,4R,5S)-4-acetylamino-5-amino3(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1). The chemical formula is C 16 H 28 N 2 O 4 (free base). The molecular weight is 312.4 for oseltamivir free base and 410.4 for oseltamivir phosphate salt. The structural formula is as follows:
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Oseltamivir Phosphate for Oral Suspension (Supplied as Powder ) Supplied as a white to off-white granular powder in a glass bottle. After constitution, the powder blend produces a white to off-white tutti-frutti–flavored oral suspension. After constitution with 55 mL of water, each bottle delivers a usable volume of 60 mL of oral suspension equivalent to 360 mg oseltamivir base (6 mg/mL) [see Dosage and Administration ( 2.5 )] (NDC 47781-384-26). Storage Store dry powder at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature]. Store constituted oral suspension under refrigeration for up to 17 days at 2º to 8ºC (36º to 46ºF). Do not freeze. Alternatively, store constituted oral suspension for up to 10 days at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Oseltamivir phosphate for oral suspension is an influenza neuraminidase inhibitor (NAI) indicated for: Treatment of acute, uncomplicated influenza A and B in patients 2 weeks of age and older who have been symptomatic for no more than 48 hours. ( 1.1 ) Prophylaxis of influenza A and B in patients 1 year and older. ( 1.2 ) Limitations of Use: Not a substitute for annual influenza vaccination. ( 1.3 ) Consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use. ( 1.3 ) Not recommended for patients with end-stage renal disease not undergoing dialysis. ( 1.3 ) 1.1 Treatment of Influenza Oseltamivir phosphate for oral suspension is indicated for the treatment of acute, uncomplicated illness due to influenza A and B infection in patients 2 weeks of age and older who have been symptomatic for no more than 48 hours. 1.2 Prophylaxis of Influenza Oseltamivir phosphate for oral suspension is indicated for the prophylaxis of influenza A and B in patients 1 year and older. 1.3 Limitations of Use Oseltamivir phosphate for oral suspension is not a substitute for early influenza vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices. Influenza viruses change over time. Emergence of resistance substitutions could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use oseltamivir phosphate for oral suspension [see Microbiology ( 12.4 )] . Oseltamivir phosphate for oral suspension is not recommended for patients with end-stage renal disease not undergoing dialysis [see Dosage and Administration ( 2.4 ) and Use in Specific Populations ( 8.6 )] .
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Treatment of influenza Adults and adolescents (13 years and older): 75 mg twice daily for 5 days ( 2.2 ) Pediatric patients 1 to 12 years of age: Based on weight twice daily for 5 days ( 2.2 ) Pediatric patients 2 weeks to less than 1 year of age: 3 mg/kg twice daily for 5 days ( 2.2 ) Renally impaired adult patients (creatinine clearance >30 to 60 mL/min): Reduce to 30 mg twice daily for 5 days ( 2.4 ) Renally impaired adult patients (creatinine clearance >10 to 30 mL/min): Reduce to 30 mg once daily for 5 days ( 2.4 ) ESRD patients on hemodialysis: Reduce to 30 mg immediately and then 30 mg after every hemodialysis cycle. Treatment duration not to exceed 5 days ( 2.4 ) ESRD patients on CAPD: Reduce to a single 30 mg dose immediately ( 2.4 ) Prophylaxis of influenza Adults and adolescents (13 years and older): 75 mg once daily for at least 10 days ( 2.3 ) Community outbreak: 75 mg once daily for up to 6 weeks ( 2.3 ) Pediatric patients 1 to 12 years of age: Based on weight once daily for 10 days ( 2.3 ) Community outbreak: Based on weight once daily for up to 6 weeks ( 2.3 ) Renally impaired adult patients (creatinine clearance >30 to 60 mL/min): Reduce to 30 mg once daily ( 2.4 ) Renally impaired adult patients (creatinine clearance >10 to 30 mL/min): Reduce to 30 mg once every other day ( 2.4 ) ESRD patients on hemodialysis: Reduce to 30 mg immediately and then 30 mg after alternate hemodialysis cycles for the recommended duration of prophylaxis ( 2.4 ) ESRD patients on CAPD: Reduce to 30 mg immediately and then 30 mg once weekly for the recommended duration of prophylaxis ( 2.4 ) 2.1 Dosage and Administration Overview Administer oseltamivir phosphate for oral suspension for the treatment of influenza in patients 2 weeks of age or older [see Dosage and Administration ( 2.2 )] or for prophylaxis of influenza in patients 1 year and older [see Dosage and Administration ( 2.3 )] using: Oseltamivir phosphate for oral suspension (supplied as a powder). This is the preferred formulation (6 mg per mL) for patients who cannot swallow capsules. Prior to use, the supplied oseltamivir phosphate for oral suspension powder must be constituted with water by the pharmacist to produce the oral suspension [see Dosage and Administration ( 2.5 )] . The oral suspension may be taken with or without food; however, tolerability may be enhanced if oseltamivir phosphate for oral suspension is taken with food. Adjust the oseltamivir phosphate for oral suspension dosage in patients with moderate or severe renal impairment [see Dosage and Administration ( 2.4 )] . 2.2 Recommended Dosage for Treatment of Influenza Initiate treatment with oseltamivir phosphate for oral suspension within 48 hours of influenza symptom onset. Adults and Adolescents (13 years of age and older) The recommended oral dosage of oseltamivir phosphate for oral suspension for treatment of influenza in adults and adolescents 13 years and older is 75 mg twice daily (12.5 mL of oral suspension twice daily) for 5 days. Pediatric Patients (2 weeks of age through 12 years of age) Table 1 displays the recommended oral dosage of oseltamivir phosphate for oral suspension for treatment of influenza in pediatric patients 2 weeks of age through 12 years of age and provides information about prescribing the formulation for oral suspension. 2.3 Recommended Dosage for Prophylaxis of Influenza Initiate post-exposure prophylaxis with oseltamivir phosphate for oral suspension within 48 hours following close contact with an infected individual. Initiate seasonal prophylaxis with oseltamivir phosphate for oral suspension during a community outbreak. Adults and Adolescents (13 years of age and older) The recommended dosage of oseltamivir phosphate for oral suspension for prophylaxis of influenza in adults and adolescents 13 years and older is 75 mg orally once daily (12.5 mL of oral suspension once daily) for at least 10 days following close contact with an infected individual and up to 6 weeks during a community outbreak. In immunocompromised patients, oseltamivir phosphate for oral suspension may be continued for up to 12 weeks [see Use in Specific Populations ( 8.9 )] . The duration of protection lasts for as long as oseltamivir phosphate for oral suspension dosing is continued. Pediatric Patients (1 year to 12 years of age) Table 1 displays the recommended oral dosage of oseltamivir phosphate for prophylaxis of influenza in pediatric patients 1 year to 12 years of age based on body weight and provides information about prescribing the formulation for oral suspension. Prophylaxis in pediatric patients is recommended for 10 days following close contact with an infected individual and up to 6 weeks during a community outbreak [see Use in Specific Populations ( 8.4 ) and Clinical Studies ( 14.2 )] . Table 1 Oseltamivir Phosphate for Oral Suspension Dosage Recommendations in Pediatric Patients for Treatment and Prophylaxis of Influenza Weight Treatment Dosage for 5 days Prophylaxis Dosage for 10 days* Volume of Oral Suspension (6 mg/mL) for each Dose † Number of Bottles of Oral Suspension to Dispense *The recommended duration for post-exposure prophylaxis is 10 days and the recommended duration for community outbreak (seasonal/pre-exposure) prophylaxis is up to 6 weeks (or up to 12 weeks in immunocompromised patients). The amount supplied (e.g., number of bottles) for seasonal prophylaxis may be greater than for post-exposure prophylaxis. †Use an oral dosing dispensing device that measures the appropriate volume in mL with the oral suspension. ‡For patients less than 1 year of age, provide an appropriate dosing device that can accurately measure and administer small volumes. Patients from 2 Weeks to less than 1 Year of Age Any weight 3 mg/kg twice daily Not applicable 0.5 mL/kg ‡ 1 bottle Patients 1 to 12 Years of Age Based on Body Weight 15 kg or less 30 mg twice daily 30 mg once daily 5 mL 1 bottle 15.1 kg to 23 kg 45 mg twice daily 45 mg once daily 7.5 mL 2 bottles 23.1 kg to 40 kg 60 mg twice daily 60 mg once daily 10 mL 2 bottles 40.1 kg or more 75 mg twice daily 75 mg once daily 12.5 mL 3 bottles 2.4 Dosage in Patients with Renal Impairment Table 2 displays the dosage recommendations for the treatment and prophylaxis of influenza in adults with various stages of renal impairment (estimated creatinine clearance of less than or equal to 90 mL per minute). Dosage modifications are recommended in adults with an estimated creatinine clearance less than or equal to 60 mL per minute [see Use in Specific Population ( 8.6 ) and Clinical Pharmacology ( 12.3 )] . Table 2 Recommended Dosage Modifications for Treatment and Prophylaxis of Influenza in Adults with Renal Impairment or End Stage Renal Disease (ESRD) on Dialysis Renal Impairment (Creatinine Clearance) Recommended Treatment Regimen* Recommended Prophylaxis Regimen† *Oral suspension can be used for 30 mg dosing. †The recommended duration for post-exposure prophylaxis is at least 10 days and the recommended duration for community outbreak (seasonal/pre-exposure) prophylaxis is up to 6 weeks (or up to 12 weeks in immunocompromised patients). ‡Data derived from studies in continuous ambulatory peritoneal dialysis (CAPD) patients. Mild (>60 to 90 mL/minute) 75 mg twice daily for 5 days 75 mg once daily Moderate (>30 to 60 mL/minute) 30 mg twice daily for 5 days 30 mg once daily Severe (>10 to 30 mL/minute) 30 mg once daily for 5 days 30 mg every other day ESRD Patients on Hemodialysis (≤10 mL/minute) 30 mg immediately and then 30 mg after every hemodialysis cycle (treatment duration not to exceed 5 days) 30 mg immediately and then 30 mg after alternate hemodialysis cycles ESRD Patients on Continuous Ambulatory Peritoneal Dialysis‡ (≤10 mL/minute) A single 30 mg dose administered immediately 30 mg immediately and then 30 mg once weekly ESRD Patients not on Dialysis Oseltamivir phosphate for oral suspension is not recommended Oseltamivir phosphate for oral suspension is not recommended 2.5 Preparation and Storage of Constituted Oseltamivir Phosphate for Oral Suspension Prior to dispensing to the patient, constitute oseltamivir phosphate for oral suspension (supplied as powder): a) Tap the closed bottle containing the supplied oseltamivir phosphate for oral suspension white to off-white powder several times to loosen the powder. b) Measure 55 mL of water in a graduated cylinder. c) Add the total amount of water for constitution to the bottle. d) Close bottle with child-resistant cap tightly and shake the closed bottle well for 15 seconds. e) Label the bottle with instructions to “Shake Well Before Use”. f) The constituted oral suspension contains 360 mg of oseltamivir base per 60 mL of volume (6 mg per mL) and is white to off-white, tutti-frutti–flavored. Use the constituted oral suspension within 17 days of preparation when stored under refrigeration, 2º to 8ºC (36º to 46ºF), or within 10 days if stored at controlled room temperature, 25ºC (77ºF). Write the expiration date of the constituted oral suspension on the bottle label. g) Ensure patients have an oral dosing dispenser that measures the appropriate volume in milliliters. Counsel patients on how to utilize the oral dosing dispenser and correctly measure the oral suspension as prescribed (see Tables 1 and 2).
