Drug Catalog - Product Detail
Ondansetron HCl Tab 8 MG 30 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0188-30 | AUROBINDO PHARMA | 30 | 8MG | TABLET |
PACKAGE FILES

Generic Name
ONDANSETRON HYDROCHLORIDE
Substance Name
ONDANSETRON HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078539
Description
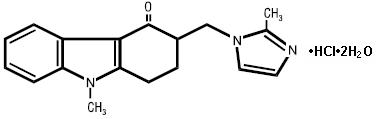
11 DESCRIPTION The active ingredient in ondansetron tablets, USP is ondansetron hydrochloride as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT 3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula: The molecular formula is C 18 H 19 N 3 O•HCl•2H 2 O, representing a molecular weight of 365.9. Ondansetron hydrochloride USP (dihydrate) is a white to off-white powder that is soluble in water and normal saline. Ondansetron tablets, USP for oral administration contain ondansetron hydrochloride USP (dihydrate) equivalent to 4 mg or 8 mg or 24 mg of ondansetron. Each film-coated tablet also contains the inactive ingredients anhydrous lactose, microcrystalline cellulose, pregelatinized starch (maize), magnesium stearate, triacetin, titanium dioxide and hypromellose. In addition 8 mg tablet also contains iron oxide yellow and 24 mg tablet also contains iron oxide red. Meets USP dissolution test 6. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Ondansetron Tablets USP, 4 mg are white to off-white, oval shaped, film-coated tablets debossed with ‘F’ on one side and ‘91’ on the other side. Bottles of 30 NDC 65862-187-30 Bottles of 500 NDC 65862-187-05 Bottles of 1,000 NDC 65862-187-99 1 x 3 Unit-dose Tablets NDC 65862-187-03 10 x 10 Unit-dose Tablets NDC 65862-187-10 Ondansetron Tablets USP, 8 mg are yellow colored, oval shaped, film-coated tablets debossed with ‘F’ on one side and ‘92’ on the other side. Bottles of 30 NDC 65862-188-30 Bottles of 500 NDC 65862-188-05 Bottles of 1,000 NDC 65862-188-99 1 x 3 Unit-dose Tablets NDC 65862-188-03 10 x 10 Unit-dose Tablets NDC 65862-188-10 Ondansetron Tablets USP, 24 mg are pink colored, oval shaped, film-coated tablets debossed with ‘C’ on one side and ‘71’ on the other side. Unit-dose Packs of 1 Tablet NDC 65862-189-11 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light.
Indications & Usage
1 INDICATIONS AND USAGE Ondansetron tablets are indicated for the prevention of nausea and vomiting associated with: highly emetogenic cancer chemotherapy, including cisplatin greater than or equal to 50 mg/m 2 initial and repeat courses of moderately emetogenic cancer chemotherapy radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen Ondansetron tablets are also indicated for the prevention of postoperative nausea and/or vomiting. Ondansetron tablets are a 5-HT 3 receptor antagonist indicated for the prevention of: nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin greater than or equal to 50 mg/m 2 (1) nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (1) nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen (1) postoperative nausea and/or vomiting (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION See full prescribing information for the recommended dosage in adults and pediatrics. (2) Patients with severe hepatic impairment: do not exceed a total daily dose of 8 mg. (2.2 , 8.6 ) 2.1 Dosage The recommended dosage regimens for adult and pediatric patients are described in Table 1 and Table 2, respectively. Corresponding doses of ondansetron tablets, ondansetron orally disintegrating tablets and ondansetron oral solution may be used interchangeably. Table 1: Adult Recommended Dosage Regimen for Prevention of Nausea and Vomiting Indication Dosage Regimen Highly Emetogenic Cancer Chemotherapy A single 24 mg dose administered 30 minutes before the start of single-day highly emetogenic chemotherapy, including cisplatin greater than or equal to 50 mg/m 2 Moderately Emetogenic Cancer Chemotherapy 8 mg administered 30 minutes before the start of chemotherapy, with a subsequent 8 mg dose 8 hours after the first dose. Then administer 8 mg twice a day (every 12 hours) for 1 to 2 days after completion of chemotherapy. Radiotherapy For total body irradiation : 8 mg administered 1 to 2 hours before each fraction of radiotherapy each day. For single high-dose fraction radiotherapy to the abdomen : 8 mg administered 1 to 2 hours before radiotherapy, with subsequent 8 mg doses every 8 hours after the first dose for 1 to 2 days after completion of radiotherapy. For daily fractionated radiotherapy to the abdomen : 8 mg administered 1 to 2 hours before radiotherapy, with subsequent 8 mg doses every 8 hours after the first dose for each day radiotherapy is given. Postoperative 16 mg administered 1 hour before induction of anesthesia. Table 2: Pediatric Recommended Dosage Regimen for Prevention of Nausea and Vomiting Indication Dosage Regimen Moderately Emetogenic Cancer Chemotherapy 12 to 17 years of age: 8 mg administered 30 minutes before the start of chemotherapy, with a subsequent 8 mg dose 8 hours after the first dose. Then administer 8 mg twice a day (every 12 hours) for 1 to 2 days after completion of chemotherapy. 4 to 11 years of age: 4 mg administered 30 minutes before the start of chemotherapy, with a subsequent 4 mg dose 4 and 8 hours after the first dose. Then administer 4 mg three times a day for 1 to 2 days after completion of chemotherapy. 2.2 Dosage in Hepatic Impairment In patients with severe hepatic impairment (Child-Pugh score of 10 or greater), do not exceed a total daily dose of 8 mg [see Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ] .
