Drug Catalog - Product Detail
OMEPRAZOLE SOD BCRB 20 1100MG CAP 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0913-02 | CIPLA USA | 30 | 20-1100MG | CAPSULE |
PACKAGE FILES


Generic Name
OMEPRAZOLE SODIUM BICARBONATE
Substance Name
OMEPRAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207476
Description
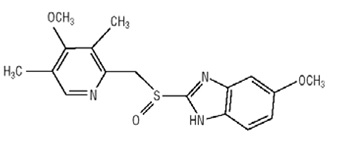
11 DESCRIPTION Omeprazole and sodium bicarbonate is a combination of omeprazole, a proton-pump inhibitor, and sodium bicarbonate, an antacid. Omeprazole is a substituted benzimidazole, 5-methoxy-2- [[(4-methoxy-3,5dimethyl-2-pyridinyl)methyl]sulfinyl]-1 H-benzimidazole, a racemic mixture of two enantiomers that inhibits gastric acid secretion. Its empirical formula is C 17H 19N 3O 3S, with a molecular weight of 345.42. The structural formula is: Omeprazole, USP is a white or almost white powder which melts with decomposition at about 155°C. Soluble in dichloromethane, practically insoluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media but has acceptable stability under alkaline conditions. Omeprazole and sodium bicarbonate is supplied as immediate-release capsules. Each capsule contains either 40 mg or 20 mg of omeprazole and 1100 mg of sodium bicarbonate with the following excipients: croscarmellose sodium and sodium stearyl fumarate. The capsules consist of gelatin and titanium dioxide. In addition the 20 mg/1100 mg capsule shell contains sodium lauryl sulfate and the 40 mg/1100 mg capsule shell contains FD&C Blue 1. The capsules are printed with edible ink containing black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, propylene glycol, potassium hydroxide, shellac and strong ammonia solution. Omeprazole and sodium bicarbonate capsules are immediate-release formulations that contain sodium bicarbonate which raises the gastric pH and thus protects omeprazole from acid degradation. STR
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Omeprazole and Sodium Bicarbonate Capsules, 20mg/1100 mg: White to off white powder filled in size “00” hard gelatin capsules with opaque white colored cap and opaque white colored body imprinted “SG” on cap and “363” on body with black ink. They are supplied as: NDC: 69097-913-02 Bottles of 30s NDC: 69097-913-12 Bottles of 500s Omeprazole and Sodium Bicarbonate Capsules, 40 mg/1100 mg: White to off white powder filled in size “00” hard gelatin capsules with opaque light blue colored cap and opaque white colored body imprinted “SG” on cap and “364” on body with black ink. They are supplied as: NDC: 69097-914-02 Bottles of 30s NDC: 69097-914-12 Bottles of 500s Storage Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed. Protect from light and moisture.
Indications & Usage
1 INDICATIONS AND USAGE Omeprazole and sodium bicarbonate capsules are indicated in adults for the: short-term treatment of active duodenal ulcer. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy. short-term treatment (4 to 8 weeks) of active benign gastric ulcer. treatment of heartburn and other symptoms associated with GERD for up to 4 weeks. short-term treatment (4 to 8 weeks) of EE due to acid-mediated GERD which has been diagnosed by endoscopy in adults. The efficacy of omeprazole and sodium bicarbonate used for longer than 8 weeks in patients with EE has not been established. If a patient does not respond to 8 weeks of treatment, an additional 4 weeks of treatment may be given. If there is recurrence of EE or GERD symptoms (e.g., heartburn), additional 4 to 8-week courses of omeprazole and sodium bicarbonate may be considered. maintenance of healing of EE due to acid-mediated GERD. Controlled studies do not extend beyond 12 months. Omeprazole and sodium bicarbonate is a proton pump inhibitor (PPI). Omeprazole and sodium bicarbonate capsules are indicated in adults for: Treatment of active duodenal ulcer ( 1 ) Treatment of active benign gastric ulcer ( 1 ) Treatment of erosive esophagitis (EE) due to acid-mediated gastroesophageal reflux disease (GERD) ( 1 ) Maintenance of healing of EE ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Indication Recommended Adult Dosage Omeprazole and sodium bicarbonate capsules Active Duodenal Ulcer 20 mg once daily for 4 weeks; some patients may require an additional 4 weeks Active Benign Gastric Ulcer 40 mg once daily for 4 to 8 weeks Treatment of Symptomatic GERD 20 mg once daily for up to 4 weeks Treatment of EE due to Acid-Mediated GERD 20 mg once daily for 4 to 8 weeks* Maintenance of Healing of EE due to Acid-Mediated GERD 20 mg once daily** * an additional 4 weeks of treatment may be given if no response; if recurrence additional 4 to 8-week courses may be considered. ** studied for 12 months. 2.1 Important Administration Instructions Omeprazole and sodium bicarbonate is available as a capsule in 20 mg and 40 mg strengths of omeprazole for adult use. All recommended doses throughout the labeling are based upon omeprazole. The sodium content of omeprazole and sodium bicarbonate capsules should be taken into consideration when prescribing this product [see Warnings and Precautions ( 5.3 )] : Omeprazole and sodium bicarbonate capsule: each 20 mg and 40 mg capsule contains 1,100 mg (13 mEq) of sodium bicarbonate. The total content of sodium in each capsule is 304 mg. Due to the sodium bicarbonate content of omeprazole and sodium bicarbonate capsules: Do not substitute two 20 mg omeprazole and sodium bicarbonate capsules with one 40 mg omeprazole and sodium bicarbonate capsule. 2.2 Dosage Regimen The recommended dosage regimen by indication in adults of omeprazole and sodium bicarbonate capsules is summarized in Table 1 . All recommended dosages are based upon omeprazole content. Table 1: Recommended Dosage Regimen of Omeprazole and Sodium Bicarbonate Capsules in Adults by Indication 1 Most patients heal within 4 weeks. Some patients may require an additional 4 weeks of therapy [See Clinical Studies (14.1)]. 2 The efficacy of omeprazole and sodium bicarbonate capsules used for longer than 8 weeks in patients with EE has not been established. If a patient does not respond to 8 weeks of treatment, an additional 4 weeks of treatment may be given. If there is recurrence of EE or GERD symptoms (e.g., heartburn), additional 4 to 8-week courses of omeprazole and sodium bicarbonate capsules may be considered. Indication Dosage of Omeprazole and Sodium Bicarbonate Capsules Treatment Duration Treatment of Active Duodenal Ulcer 20 mg once daily 4 weeks 1,2 Treatment of Active Benign Gastric Ulcer 40 mg once daily 4 to 8 weeks Treatment of Symptomatic GERD 20 mg once daily Up to 4 weeks Treatment of EE due to Acid-Mediated GERD 20 mg once daily 4 to 8 weeks 2 Maintenance of Healing of EE due to Acid-Mediated GERD 20 mg once daily Controlled studies do not extend beyond 12 months. 2.3 Preparation and Administration Omeprazole and Sodium Bicarbonate Capsules Swallow capsules intact with water. Do not open the capsule and do not administer with liquids other than water. Take on an empty stomach at least one hour before a meal [see Clinical Pharmacology ( 12.3 )].
