Drug Catalog - Product Detail
NYSTATIN ORAL SUSPENSION SUSP 500,000/5ML 10X10 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00121-4810-00 | PHARMACEUTICAL ASSOCIATES | 5 | 100000UNIT/ML | NA |
PACKAGE FILES


Generic Name
NYSTATIN
Substance Name
NYSTATIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203621
Description
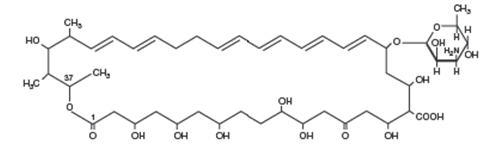
DESCRIPTION Nystatin is an antimycotic polyene antibiotic obtained from Streptomyces noursei . Structural formula: C 47 H 75 NO 17 MW 926.13 Nystatin Oral Suspension USP, for oral administration, contains 100,000 USP Nystatin Units per mL. Inactive ingredients: alcohol (≤ 1% v/v), artificial peppermint flavor, cherry flavor, citric acid, D&C Yellow No. 10, FD&C Red No. 40, glycerin, magnesium aluminum silicate, methylparaben, potassium phosphate dibasic, propylene glycol, propylparaben, purified water and sucrose. nystatin chemical structure
How Supplied
HOW SUPPLIED Nystatin Oral Suspension USP, 100,000 USP Nystatin Units per mL, is available in a cherry, peppermint flavored, light creamy yellow, ready-to-use suspension, supplied in the following oral dosage forms: NDC 0121-0810-02: 2 fl oz (60mL) bottle with calibrated dropper NDC 0121-0810-16: 16 fl oz (473mL) bottle NDC 0121-4810-05: 5mL unit dose cup NDC 0121-4810-40: Case contains 40 unit dose cups of 5mL (0121-4810-05) packaged in 4 trays of 10 unit dose cups each. Storage Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Avoid freezing.
Indications & Usage
INDICATIONS AND USAGE Nystatin oral suspension is indicated for the treatment of candidiasis in the oral cavity.
Dosage and Administration
DOSAGE AND ADMINISTRATION INFANTS: 2 mL (200,000 units) four times daily (in infants and young children, use dropper to place one-half of dose in each side of mouth and avoid feeding for 5 to 10 minutes). NOTE: Limited clinical studies in premature and low birth weight infants indicate that 1 mL four times daily is effective. CHILDREN AND ADULTS: 4 to 6 mL (400,000 to 600,000 units) four times daily (one-half of dose in each side of mouth). The preparation should be retained in the mouth as long as possible before swallowing. Continue treatment for at least 48 hours after perioral symptoms have disappeared and cultures demonstrate eradication of Candida albicans .
