Drug Catalog - Product Detail
NORGESTIMATE/ETHINYL ESTRADIOL(TRI-PREVIFEM) TB 0.180 mg/0.035 mg; 0.215 mg/0.035 mg; 0.250 mg/0.035 mg 6X28
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00603-7663-17 | PAR PHARMACEUTICAL | 28 | 0.18/0.215/0.25MG-35 MCG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
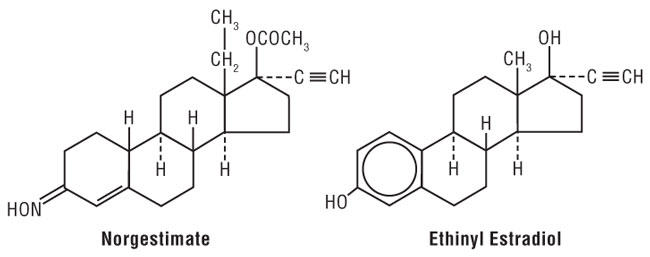
11 DESCRIPTION Each of the following products is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol. Norgestimate is designated as (18,19-Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-, oxime,(17α)-(+)-) and ethinyl estradiol is designated as (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Previfem ® Each active blue tablet contains 0.25 mg of norgestimate and 0.035 mg of ethinyl estradiol. Inactive ingredients include FD&C Blue No. 1 HT Aluminum Lake, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, and pregelatinized starch. Each light-green placebo tablet contains only inert ingredients, as follows: FD&C Blue No. 2, hypromellose, iron oxide yellow, lactose monohydrate, magnesium stearate, polyethylene glycol, and pregelatinized starch. Tri-Previfem ® Each active white tablet contains 0.18 mg of norgestimate and 0.035 mg of ethinyl estradiol. Inactive ingredients include hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, and pregelatinized starch. Each active light-blue tablet contains 0.215 mg of norgestimate and 0.035 mg of ethinyl estradiol. Inactive ingredients include FD&C Blue No. 1 Aluminum Lake, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, and pregelatinized starch. Each active blue tablet contains 0.25 mg of norgestimate and 0.035 mg of ethinyl estradiol. Inactive ingredients include FD&C Blue No. 1 Aluminum Lake, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, and pregelatinized starch. Each light-green placebo tablet contains only inert ingredients, as follows: FD&C Blue No. 2, hypromellose, iron oxide yellow, lactose monohydrate, magnesium stearate, polyethylene glycol, and pregelatinized starch. Chemical structure of Norgestimate and Ethinyl Estradiol
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Previfem ® Previfem ® (norgestimate and ethinyl estradiol tablets USP) is packaged in cartons of 6 blister pack tablet dispensers containing 28 tablets as follows: 21 blue tablets containing 0.25 mg of norgestimate and 0.035 mg of ethinyl estradiol which are round, unscored, film-coated tablets debossed with "93" and "748" on each side and 7 light-green, round, film-coated tablets debossed with "93" and "743" containing inert ingredients. Blister pack tablet dispenser NDC 0603-7642-01. Boxes of 6 blister pack tablet dispensers NDC 0603-7642-17. Tri-Previfem ® Tri-Previfem ® (norgestimate and ethinyl estradiol tablets USP) is packaged in cartons of 6 blister pack tablet dispensers, each blister pack tablet dispenser contains 28 tablets as follows: Each white tablet contains 0.18 mg of norgestimate and 0.035 mg of ethinyl estradiol. Each light-blue tablet contains 0.215 mg of norgestimate and 0.035 mg of ethinyl estradiol. Each blue tablet contains 0.25 mg of norgestimate and 0.035 mg of ethinyl estradiol. Each light-green tablet contains inert ingredients. The white tablets are round, unscored film-coated, imprinted with "93" on one side and "746" on the other side; the light-blue tablets are round, unscored film-coated, imprinted with "93" on one side and "747" on the other side; the blue tablets are round, unscored film-coated, imprinted with "93" on one side and "748" on the other side; the light-green tablets are round, film-coated, imprinted with "93" on one side and "743" on the other side. Blister pack tablet dispenser NDC 0603-7663-01. Boxes of 6 blister pack tablet dispensers NDC 0603-7663-17. Keep out of reach of children. 16.2 Storage Conditions Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] . Protect from light.
Indications & Usage
1 INDICATIONS AND USAGE Previfem ® (norgestimate/ethinyl estradiol tablets USP) and Tri-Previfem ® (norgestimate/ethinyl estradiol tablets USP) are estrogen/progestin COCs, indicated for use by women to prevent pregnancy. ( 1.1 ) Tri-Previfem ® (norgestimate/ethinyl estradiol tablets USP) is also indicated for the treatment of moderate acne vulgaris in females at least 15 years of age, who have no known contraindications to oral contraceptive therapy and have achieved menarche. Tri-Previfem ® (norgestimate/ethinyl estradiol tablets USP) should be used for the treatment of acne only if the patient desires an oral contraceptive for birth control. ( 1.2 ) 1.1 Oral Contraceptive Previfem ® (norgestimate/ethinyl estradiol tablets USP) and Tri-Previfem ® (norgestimate/ethinyl estradiol tablets USP) are indicated for use by females of reproductive potential to prevent pregnancy [see Clinical Studies (14) ] . 1.2 Acne Tri-Previfem ® (norgestimate/ethinyl estradiol tablets USP) is indicated for the treatment of moderate acne vulgaris in females at least 15 years of age, who have no known contraindications to oral contraceptive therapy and have achieved menarche. Previfem ® (norgestimate/ethinyl estradiol tablets USP) should be used for the treatment of acne only if the patient desires an oral contraceptive for birth control [see Clinical Studies (14) ] .
Dosage and Administration
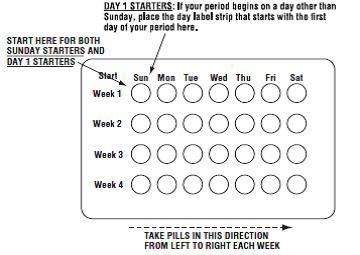
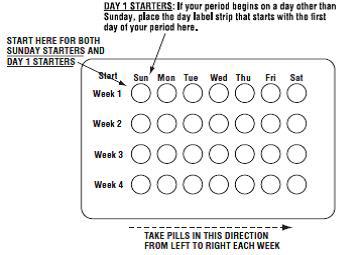
2 DOSAGE AND ADMINISTRATION • Take one tablet daily by mouth at the same time every day. ( 2.2 ) • Take tablets in the order directed on the blister pack. ( 2.2 ) • Do not skip or delay tablet intake. ( 2.2 ) 2.1 How to Start Previfem ® (norgestimate/ethinyl estradiol tablets) or Tri-Previfem ® (norgestimate/ethinyl estradiol tablets) Previfem ® (norgestimate/ethinyl estradiol tablets USP) and Tri-Previfem ® (norgestimate/ethinyl estradiol tablets USP) are dispensed in a blister pack tablet dispenser [see How Supplied/Storage and Handling (16) ]. Previfem ® (norgestimate/ethinyl estradiol tablets USP) and Tri-Previfem ® (norgestimate/ethinyl estradiol tablets USP) may be started using either a Day 1 start or a Sunday start (see Table 1). For the first cycle of a Sunday Start regimen, an additional method of contraception should be used until after the first 7 consecutive days of administration. 2.2 How to Take Previfem ® or Tri-Previfem ® Table 1: Instructions for Administration of Previfem ® or Tri-Previfem ® Starting COCs in women not currently using hormonal contraception (Day 1 Start or Sunday Start) Important: Consider the possibility of ovulation and conception prior to initiation of this product. Tablet Color: Previfem ® active tablets are blue (Day 1 to Day 21). Tri-Previfem ® active tablets are white (Day 1 to Day 7), light blue (Day 8 to Day 15) and blue (Day 16 to Day 21). Previfem ® and Tri-Previfem ® both have light-green inactive tablets (Day 22 to Day 28). Day 1 Start: Take first active tablet without regard to meals on the first day of menses. Take subsequent active tablets once daily at the same time each day for a total of 21 days. Take one light-green inactive tablet daily for 7 days and at the same time of day that active tablets were taken. Begin each subsequent pack on the same day of the week as the first cycle pack (i.e., on the day after taking the last inactive tablet) Sunday Start: Take first active tablet without regard to meals on the first Sunday after the onset of menses. Due to the potential risk of becoming pregnant, use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of the patient's first cycle pack of Previfem ® or Tri-Previfem ® . Take subsequent active tablets once daily at the same time each day for a total of 21 days. Take one light-green inactive tablet daily for the following 7 days and at the same time of day that active tablets were taken. Begin each subsequent pack on the same day of the week as the first cycle pack (i.e., on the Sunday after taking the last inactive tablet) and additional non-hormonal contraceptive is not needed. Switching to Previfem ® or Tri-Previfem ® from another oral contraceptive Start on the same day that a new pack of the previous oral contraceptive would have started. Switching from another contraceptive method to Previfem ® or Tri-Previfem ® Start Previfem ® or Tri-Previfem ® : Transdermal patch On the day when next application would have been scheduled Vaginal ring On the day when next insertion would have been scheduled Injection On the day when next injection would have been scheduled Intrauterine contraceptive On the day of removal If the IUD is not removed on first day of the patient's menstrual cycle, additional non-hormonal contraceptive (such as condoms and spermicide) is needed for the first seven days of the first cycle pack. Implant On the day of removal Complete instructions to facilitate patient counseling on proper tablet usage are located in the FDA-Approved Patient Labeling. Starting Previfem ® and Tri-Previfem ® after Abortion or Miscarriage First-trimester After a first-trimester abortion or miscarriage, Previfem ® or Tri-Previfem ® may be started immediately. An additional method of contraception is not needed if Previfem ® or Tri-Previfem ® is started immediately. If Previfem ® or Tri-Previfem ® is not started within 5 days after termination of the pregnancy, the patient should use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of her first cycle pack of Previfem ® or Tri-Previfem ® . Second-trimester Do not start until 4 weeks after a second-trimester abortion or miscarriage, due to the increased risk of thromboembolic disease. Start Previfem ® or Tri-Previfem ® , following the instructions in Table 1 for Day 1 or Sunday start, as desired. If using Sunday start, use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of the patient's first cycle pack of Previfem ® or Tri-Previfem ® [see Contraindications (4) , Warnings and Precautions (5.1) , and FDA-Approved Patient Labeling ]. Starting Previfem ® or Tri-Previfem ® after Childbirth Do not start until 4 weeks after delivery, due to the increased risk of thromboembolic disease. Start contraceptive therapy with Previfem ® or Tri-Previfem ® following the instructions in Table 1 for women not currently using hormonal contraception. Previfem ® or Tri-Previfem ® are not recommended for use in lactating women [see Use in Specific Populations (8.3) ] . If the woman has not yet had a period postpartum, consider the possibility of ovulation and conception occurring prior to use of Previfem ® or Tri-Previfem ® [see Contraindications (4) , Warnings and Precautions (5.1) , Use in Specific Populations (8.1 and 8.3) , and FDA-Approved Patient Labeling ]. Previfem ® and Tri-Previfem ® come in a blister pack pill dispenser. Read the instructions below for using the blister pack pill dispenser. The blister package consists of three parts, the calendar label, the sleeve and the blister pack containing 28 individually sealed pills. Note that the pills are arranged in four numbered rows of 7 pills, with the pre-printed days of the week printed above them. Refer to the sample of the blister pack below: Previfem ® consists of 21 blue "active" birth control pills and 7 light green "reminder" pills. Tri-Previfem ® consists of 7 white "active" pills, 7 light-blue "active" pills, 7 blue "active" pills and 7 light green "reminder" pills. There are two ways to start taking birth-control pills, Sunday Start or Day 1 Start. How to use Blister Cards for the 28 tablets 1. If Sunday Start, the patient discards the stickers and takes the first active pill on the first Sunday after their menstrual period begins. Due to the potential risk of becoming pregnant, use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of the patient's first cycle pack of Previfem ® or Tri-Previfem ® . 2. If Day 1 Start, the patient picks the Days of the Week Sticker that starts the first day of their period. When the patient has picked the right sticker, they need to throw away the others and place the sticker on the blister card over the preprinted days of the week and make sure it lines up with the pills. 3. The patient removes the first pill by pushing down on the pill and waits 24 hours to take their next pill. The patient continues to take one pill each day until all the pills have been taken. 4. The pill should be taken at the same time each day. 5. After taking the last pill, the patient starts a new blister pack the very next day, no matter when their next period starts. 6. The patient should take the pills in each new package as before and start with the pill on the first row and take one pill each day, left to right, until the last pill has been taken. Blister card guide 2.3 Missed Tablets Table 2: Instructions for Missed Previfem ® or Tri-Previfem ® Tablets If one active tablet is missed in Weeks 1, 2, or 3 Take the tablet as soon as possible. Continue taking one tablet a day until the pack is finished. If two active tablets are missed in Week 1 or Week 2 Take the two missed tablets as soon as possible and the next two active tablets the next day. Continue taking one tablet a day until the pack is finished. Additional non-hormonal contraception (such as condoms and spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. If two active tablets are missed in the third week or three or more active tablets are missed in a row in Weeks 1, 2, or 3 Day 1 start : Throw out the rest of the pack and start a new pack that same day. Sunday start : Continue taking one tablet a day until Sunday, then throw out the rest of the pack and start a new pack that same day. Additional non-hormonal contraception (such as condoms and spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. 2.4 Advice in Case of Gastrointestinal Disturbances In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting or diarrhea occurs within 3 to 4 hours after taking an active tablet, handle this as a missed tablet [see FDA-Approved Patient Labeling ] . 2.5 Tri-Previfem ® Use for Acne The timing of initiation of dosing with Tri-Previfem ® for acne should follow the guidelines for use of Tri-Previfem ® as an oral contraceptive. Consult the DOSAGE AND ADMINISTRATION section (2.1) for instructions.
