Drug Catalog - Product Detail
NORETHINDRONE ACETATE/ETHINYL ESTRADIOL TB 0.5MG/2.5MCG 3X28
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0656-29 | GLENMARK PHARMACEUTICALS | 28 | 0.5-2.5MG-MCG | TABLET |
PACKAGE FILES




Generic Name
NORETHINDRONE ACETATE AND ETHINYL ESTRADIOL
Substance Name
ETHINYL ESTRADIOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203038
Description
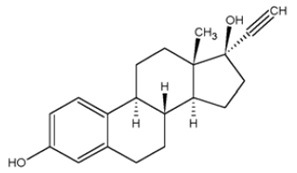
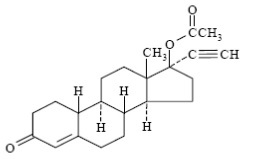
11 DESCRIPTION Norethindrone Acetate and Ethinyl Estradiol Tablets, USP is a continuous dosage regimen of a progestin-estrogen combination for oral administration. The following two strengths of Norethindrone Acetate and Ethinyl Estradiol Tablets, USP are available as: Norethindrone Acetate and Ethinyl Estradiol Tablets USP (0.5 mg/2.5 mcg): Each light pink to pink, round, flat-faced beveled edge, uncoated tablet contains 0.5 mg norethindrone acetate, USP and 2.5 mcg ethinyl estradiol, USP. Norethindrone Acetate and Ethinyl Estradiol Tablets USP (1 mg/5 mcg): Each white to off-white, round, flat-faced beveled edge, uncoated tablet contains 1 mg norethindrone acetate, USP and 5 mcg ethinyl estradiol, USP. Each tablet also contains the following inactive ingredients: calcium stearate, cornstarch, lactose monohydrate, microcrystalline cellulose, povidone and vitamin E and talc. The 0.5 mg/2.5 mcg light pink to pink tablet also contains D&C red no. 30. The structural formulas are as follows Ethinyl Estradiol, USP [19-Nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol] Molecular Weight: 296.40 Molecular Formula: C 20 H 24 O 2 Norethindrone Acetate USP [19-Norpregn-4-en-20-yn-3-one, 17-(acetyloxy)-, (17 α)] Molecular Weight: 340.46 Molecular Formula: C 22 H 28 O 3 eestructure noreestructure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Norethindrone Acetate and Ethinyl Estradiol Tablets, USP are available in the following strengths and package sizes: Norethindrone Acetate and Ethinyl Estradiol Tablets, USP 0.5 mg/2.5 mcg, are light pink to pink, round, flat-faced, beveled edge, uncoated, tablets debossed with “D5” on one side and plain on the other side. Each tablet contains 0.5 mg norethindrone acetate, USP and 2.5 mcg ethinyl estradiol, USP and are available as: NDC 68462-656-90 Bottle of 90 Tablets NDC 68462-656-29 1 carton containing 3 individual foil pouches. Each pouch contains 1 blister of 28 tablets Norethindrone Acetate and Ethinyl Estradiol Tablets, USP 1 mg/5 mcg are white to off-white, round, flat-faced, beveled edge, uncoated tablets debossed with “D6” on one side plain on the other side. Each tablet contains 1 mg norethindrone acetate, USP and 5 mcg ethinyl estradiol, USP and are available as: NDC 68462-657-90 Bottle of 90 Tablets NDC 68462-657-29 1 carton containing 3 individual foil pouches. Each pouch contains 1 blister of 28 tablets 16.2 Storage and Handling Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Keep norethindrone acetate and ethinyl estradiol tablets out of the reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Norethindrone acetate and ethinyl estradiol tablets are a combination of an estrogen and progestin indicated in a woman with a uterus for: • Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause ( 1.1 ) • Prevention of Postmenopausal Osteoporosis ( 1.2 ) 1.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause 1.2 Prevention of Postmenopausal Osteoporosis Limitation of Use When prescribing solely for the prevention of postmenopausal osteoporosis, first consider the use of non-estrogen medications. Consider estrogen therapy only for women at significant risk of osteoporosis.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Use estrogen, alone or in combination with a progestogen, at the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Re-evaluate postmenopausal women periodically as clinically appropriate to determine whether treatment is still necessary. • One tablet orally once daily ( 2.1 , 2.2 ) 2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause Take a single norethindrone acetate and ethinyl estradiol tablet, orally once daily. 2.2 Prevention of Postmenopausal Osteoporosis Take a single norethindrone acetate and ethinyl estradiol tablet, orally once daily
