Drug Catalog - Product Detail
NORDESTRAL/ETHINYL ESTRADIOL (CRYSELLE) TB 0.3/0.03MG 6X28
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00555-9049-58 | TEVA PHARMACEUTICALS USA | 28 | 0.3-30MG-MCG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
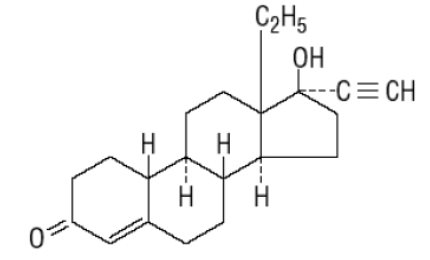
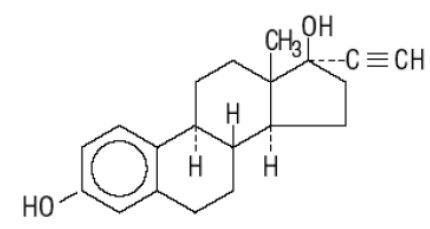
DESCRIPTION Cryselle ® is a combination oral contraceptive containing the progestational compound norgestrel, USP and the estrogenic compound ethinyl estradiol, USP. Norgestrel is designated as (2) (±)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one and ethinyl estradiol is designated as (19-nor-17α-pregna-1,3,5 (10)-trien-20-yne-3,17-diol). Each white active Cryselle tablet contains 0.3 mg norgestrel, USP and 0.03 mg ethinyl estradiol, USP. The inactive ingredients present are hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol and pregelatinized corn starch. The light-green inactive tablets also contain D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake and FD&C Yellow No. 6 Aluminum Lake. Norgestrel, USP C 21 H 28 O 2 MW: 312.45 Ethinyl Estradiol, USP C 20 H 24 O 2 MW: 296.40 Norgestrel Strustural Formula Ethinyl Estradiol Structural Formula
How Supplied
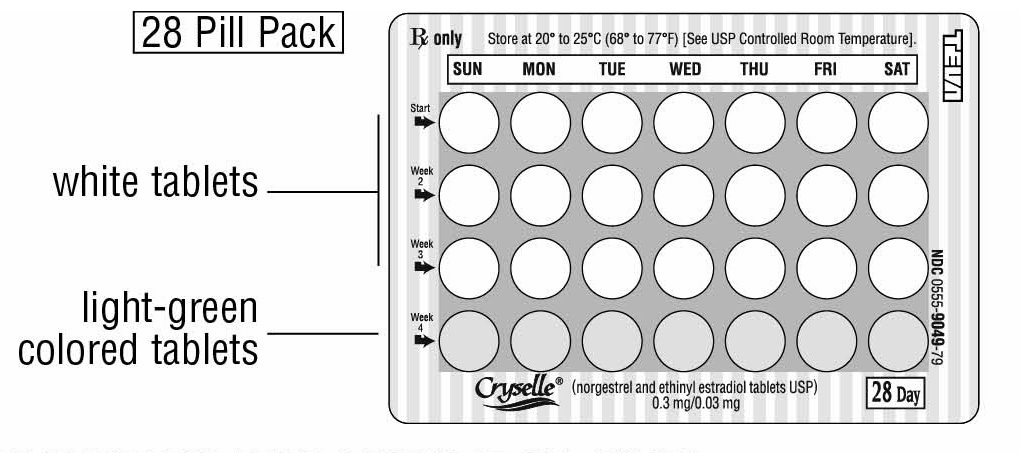
HOW SUPPLIED Cryselle ® (norgestrel and ethinyl estradiol tablets USP), 0.3 mg/0.03 mg are available in packages of 6 blister card dispensers (NDC 0555-9049-58), each containing 28 tablets as follows: 21 active, white, round, film-coated, biconvex tablets debossed with dp on one side and 543 on the other side and 7 inert, round, light-green colored, uncoated tablets debossed dp and 331. Store at 20º to 25°C (68° to 77º F) [See USP Controlled Room Temperature]. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. Teva Pharmaceuticals USA, Inc. North Wales, PA 19454 Rev. G 7/2022
Indications & Usage
INDICATIONS AND USAGE Cryselle is indicated for use by females of reproductive potential to prevent pregnancy. In a study of 1,287 women with a total of 11,085 cycles or 852.7 women-years of usage, the pregnancy rate in women age 15 to 40 years was approximately 1 pregnancy per 100 women-years of use.
Dosage and Administration
