Drug Catalog - Product Detail
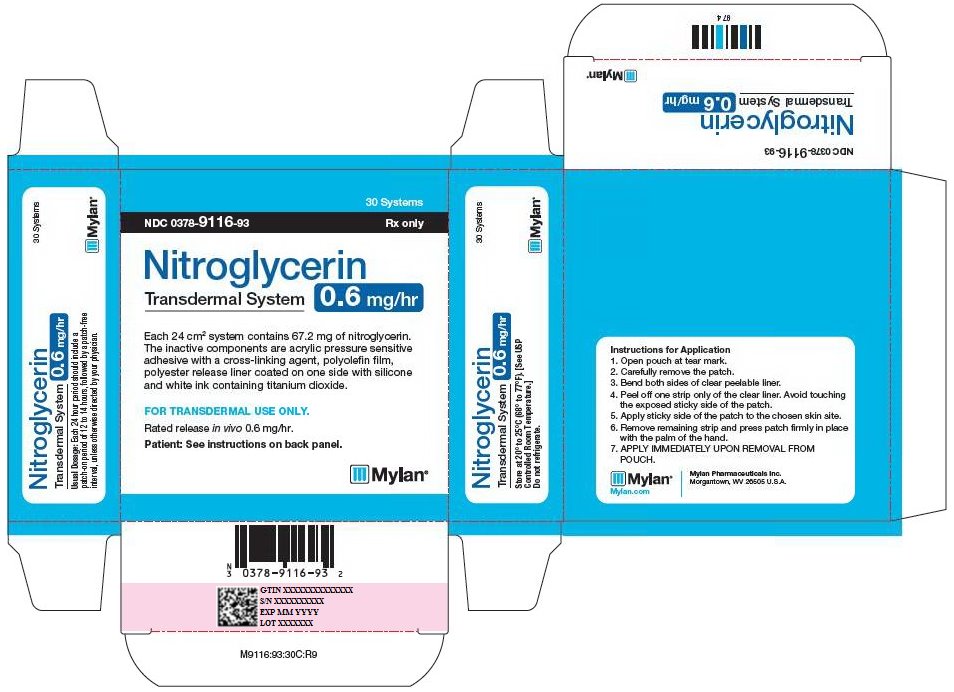
NITROGLYCERIN TRANSDERMAL PATCH PATCH .6MG/HR 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-9116-93 | MYLAN | 30 | 0.6MG/HR | TRANSDERMAL SYSTEM |
PACKAGE FILES
Generic Name
NITROGLYCERIN
Substance Name
NITROGLYCERIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
TRANSDERMAL
Application Number
ANDA074559
Description
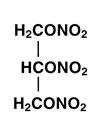
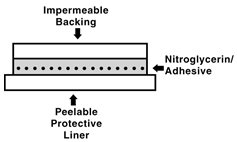
DESCRIPTION Nitroglycerin is 1,2,3-propanetriol, trinitrate, an organic nitrate whose structural formula is: and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins. The nitroglycerin transdermal system is a flat unit designed to provide continuous controlled release of nitroglycerin through intact skin. The rate of release of nitroglycerin is linearly dependent upon the area of the applied system; each cm 2 of applied system delivers approximately 0.026 mg of nitroglycerin per hour. Thus, the 4 cm 2 , 8 cm 2 , 16 cm 2 and 24 cm 2 systems deliver approximately 0.1 mg, 0.2 mg, 0.4 mg and 0.6 mg of nitroglycerin per hour, respectively. The remainder of the nitroglycerin in each system serves as a reservoir and is not delivered in normal use. After 12 hours, for example, each system has delivered approximately 11% of its original content of nitroglycerin. The nitroglycerin transdermal system comprises two layers as shown below. Proceeding from the visible surface towards the surface attached to the skin, these layers are: 1) a polyolefin film backing layer that is impermeable to nitroglycerin and is printed with the name of the drug and strength; 2) nitroglycerin in an acrylic pressure sensitive adhesive. Prior to use, a peelable polyester release liner, which is coated on one side with silicone, is removed from the adhesive surface. Each unit is sealed in a foil-lined pouch. Cross section of the system: Nitroglycerin Structural Formula Patch Cross Section
How Supplied
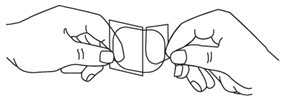
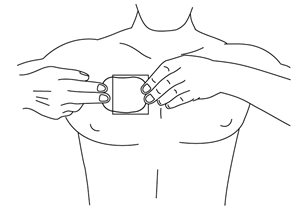
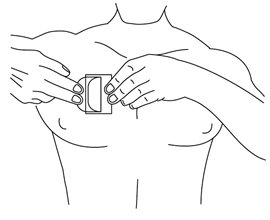
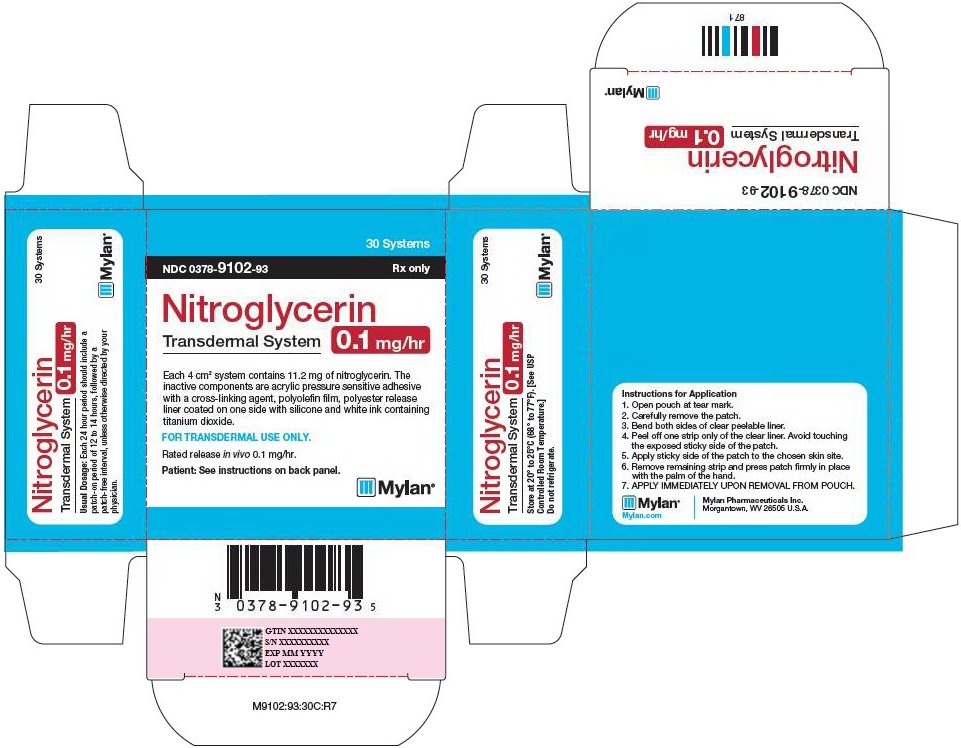
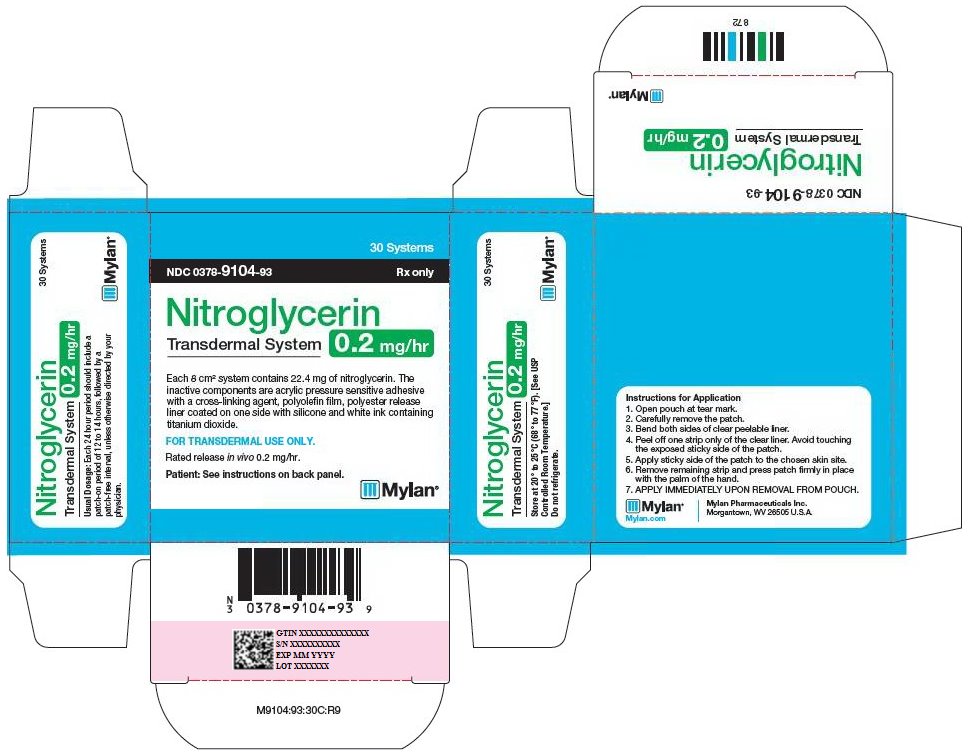
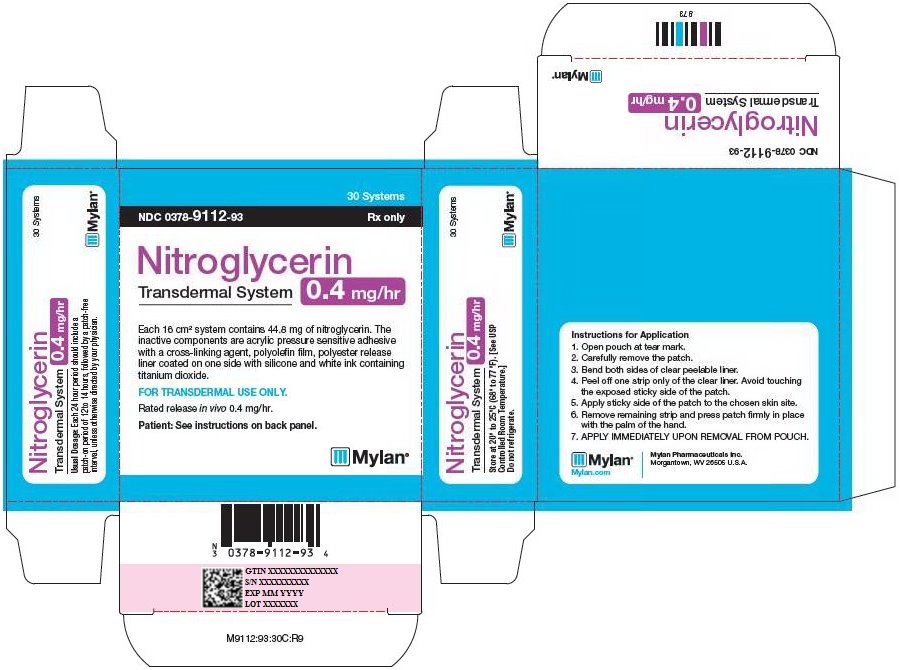
HOW SUPPLIED Nitroglycerin Transdermal System 0.1 mg/hr is a translucent rectangular patch with rounded corners (registered imprint ‘Nitroglycerin 0.1 mg/hr’ in white ink) affixed to a clear, peelable liner, and is supplied in a foil-lined pouch. NDC 0378-9102-93 Carton of 30 Systems Nitroglycerin Transdermal System 0.2 mg/hr is a translucent rectangular patch with rounded corners (registered imprint ‘Nitroglycerin 0.2 mg/hr’ in white ink) affixed to a clear, peelable liner, and is supplied in a foil-lined pouch. NDC 0378-9104-93 Carton of 30 Systems Nitroglycerin Transdermal System 0.4 mg/hr is a translucent rectangular patch with rounded corners (registered imprint ‘Nitroglycerin 0.4 mg/hr’ in white ink) affixed to a clear, peelable liner, and is supplied in a foil-lined pouch. NDC 0378-9112-93 Carton of 30 Systems Nitroglycerin Transdermal System 0.6 mg/hr is a translucent rectangular patch with rounded corners (registered imprint ‘Nitroglycerin 0.6 mg/hr’ in white ink) affixed to a clear, peelable liner, and is supplied in a foil-lined pouch. NDC 0378-9116-93 Carton of 30 Systems Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Do not refrigerate. Do not store outside of the protective package. Apply immediately upon removal from the protective package. Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A. REVISED NOVEMBER 2014 NTG:R14
Indications & Usage
INDICATIONS AND USAGE Transdermal nitroglycerin is indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of transdermal nitroglycerin is not sufficiently rapid for this product to be useful in aborting an acute attack.
Dosage and Administration
DOSAGE AND ADMINISTRATION The suggested starting dose is between 0.2 mg/hr and 0.4 mg/hr. Doses between 0.4 mg/hr and 0.8 mg/hr have shown continued effectiveness for 10 to 12 hours daily for at least one month (the longest period studied) of intermittent administration. Although the minimum nitrate-free interval has not been defined, data show that a nitrate-free interval of 10 to 12 hours is sufficient (see CLINICAL PHARMACOLOGY ). Thus, an appropriate dosing schedule for nitroglycerin patches would include a daily patch-on period of 12 to 14 hours and a daily patch-off period of 10 to 12 hours. Although some well controlled clinical trials using exercise tolerance testing have shown maintenance of effectiveness when patches are worn continuously, the large majority of such controlled trials have shown the development of tolerance (i.e., complete loss of effect) within the first 24 hours after therapy was initiated. Dose adjustment, even to levels much higher than generally used, did not restore efficacy. PATIENT INSTRUCTIONS FOR APPLICATION OF SYSTEM A patient leaflet is supplied with each carton.
