Drug Catalog - Product Detail
NEOMYCIN/POLYMYXIN B/HC OTIC SUSP SUSP 5/10/1MG/ML 10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0635-62 | BAUSCH HEALTH | 10 | 3.5-10000-1 | SUSPENSION |
PACKAGE FILES
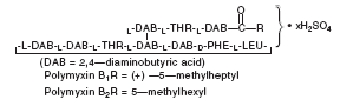

Generic Name
Substance Name
Product Type
Route
Application Number
Description
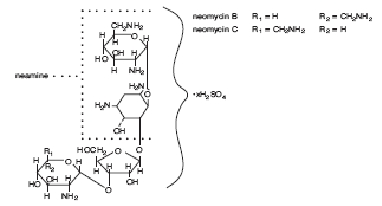
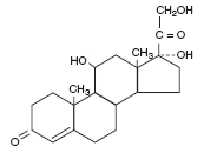
DESCRIPTION: Neomycin and Polymyxin B Sulfates and Hydrocortisone Otic Suspension USP is a sterile antibacterial and anti-inflammatory suspension for otic use. Neomycin Sulfate is the sulfate salt of neomycin B and C, which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae). It has a potency equivalent of not less than 600 micrograms of neomycin standard per milligram, calculated on an anhydrous basis. The structural formulae are: Polymyxin B Sulfate is the sulfate salt of polymyxin B 1 and B 2 , which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per milligram, calculated on an anhydrous basis. The structural formulae are: Hydrocortisone 11β,17, 21-trihydroxypregn-4-ene-3,20-dione, is an anti-inflammatory hormone. Its structural formula is: C 21 H 30 O 5 Mol. Wt. 362.47 Each mL Contains: ACTIVES: Hydrocortisone, 10 mg (1%), Neomycin Sulfate equivalent to 3.5 mg neomycin base, Polymyxin B Sulfate, equal to 10,000 polymyxin B units; INACTIVES: Propylene Glycol, Cetyl Alcohol (0.9%), Polysorbate 80, Purified Water. Sulfuric Acid may be added to adjust pH (3.0 - 7.0). PRESERVATIVE ADDED: Thimerosal 0.01%. Neomycin Sulfate (structural formula) Polymyxin B Sulfate (structural formula) Hydrocortisone (structural formula)
How Supplied
HOW SUPPLIED: Neomycin and Polymyxin B Sulfates and Hydrocortisone Otic Suspension USP is supplied in a white plastic dropper bottle in the following size: 10 mL bottles - Prod. No. 06509 Storage: Store at 15°-25°C (59°-77°F). FOR OTIC USE ONLY DO NOT USE IF IMPRINTED NECKBAND IS NOT INTACT. KEEP OUT OF REACH OF CHILDREN.
Indications & Usage
INDlCATlONS AND USAGE: For the treatment of superficial bacterial infections of the external auditory canal caused by organisms susceptible to the action of the antibiotics, and for the treatment of infections of mastoidectomy and fenestration cavities caused by organisms susceptible to the antibiotics.
Dosage and Administration
DOSAGE AND ADMlNlSTRATlON: Therapy with this product should be limited to 10 consecutive days. The external auditory canal should be thoroughly cleansed and dried with a sterile cotton applicator. For adults, 4 drops of the suspension should be instilled into the affected ear 3 to 4 times daily. For infants and children, 3 drops are suggested because of the smaller capacity of the ear canal. The patient should lie with the affected ear upward and then the drops should be instilled. This position should be maintained for 5 minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear. If preferred, a cotton wick may be inserted into the canal and then the cotton may be saturated with the suspension. This wick should be kept moist by adding further suspension every 4 hours. The wick should be replaced at least once every 24 hours. SHAKE WELL BEFORE USING.
