Drug Catalog - Product Detail
NADOLOL TAB 80 MG 100 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68382-0734-01 | ZYDUS PHARMACEUTICALS (USA) | 100 | 80MG | TABLET |
PACKAGE FILES


Generic Name
NADOLOL
Substance Name
NADOLOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA207761
Description
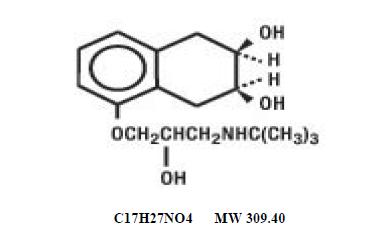
DESCRIPTION Nadolol is a synthetic nonselective beta-adrenergic receptor blocking agent designated chemically as 1-( tert -butylamino)-3-[(5,6,7,8-tetrahydro- cis -6,7-dihydroxy-1- naphthyl)oxy]-2-propanol. Structural formula: Nadolol, USP is a white to off-white, practically odorless crystalline powder. It is freely soluble in alcohol and in methanol, soluble in water at pH 2, slightly soluble in chloroform,,methylenechloride, isopropylalcohol and in water(between pH 7 and pH 10); insoluble in acetone, benzene, ether, hexane and trichloroethane. Nadolol tablets, USP are available for oral administration as 20 mg, 40 mg and 80 mg tablets. Inactive ingredients: anhydrous citric acid, colloidal silicon dioxide, corn starch, magnesium stearate, microcrystalline cellulose, povidone, and sodium starch glycolate. Additionally each 40 mg tablet contains ferric oxide yellow and each 80 mg tablet contains FD&C blue 2. Nadolol tablets
How Supplied
HOW SUPPLIED 20 mg tablets: White to off-white colored, round shaped, flat faced, beveled edge uncoated tablets, debossed with ''N" on upper half of the breakline and "20" on lower half of the breakline on one side and plain on other side and are supplied as : NDC 68382-732-16 in bottle of 90 tablets with child-resistant closure NDC 68382-732-01 in bottle of 100 tablets with child-resistant closure NDC 68382-732-10 in bottle of 1000 tablets NDC 68382-732-77 in cartons of 100 tablets (10 x 10 unit-dose) 40 mg tablets: Light yellow colored, round shaped, flat faced, beveled edge uncoated tablets, debossed with "N" on upper half of the breakline and "40" on lower half of the breakline on one side and plain on the other side and are supplied as : NDC 68382-733-16 in bottle of 90 tablets with child-resistant closure NDC 68382-733-01 in bottle of 100 tablets with child-resistant closure NDC 68382-733-10 in bottle of 1000 tablets NDC 68382-733-77 in cartons of 100 tablets (10 x 10 unit-dose) 80 mg tablets: Light blue colored, spotted, round shaped, flat faced, beveled edge uncoated tablets debossed with "N" on upper half of the breakline and "80" on lower half of the breakline on one side and plain on the other side and are supplied as : NDC 68382-734-16 in bottle of 90 tablets with child-resistant closure NDC 68382-734-01 in bottle of 100 tablets with child-resistant closure NDC 68382-734-10 in bottle of 1000 tablets NDC 68382-734-77 in cartons of 100 tablets (10 x 10 unit-dose) STORAGE Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Avoid excessive heat. Protect from light. Keep bottle tightly closed. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. Manufactured by: Zydus Lifesciences Ltd. Ahmedabad, India. Distributed by: Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534 Rev.: 01/24
Indications & Usage
INDICATIONS AND USAGE Angina Pectoris Nadolol tablets are indicated for the long-term management of patients with angina pectoris. Hypertension Nadolol tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with nadolol. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g. on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Nadolol tablets may be used alone or in combination with other antihypertensive agents, especially thiazide-type diuretics.
Dosage and Administration
DOSAGE AND ADMINISTRATION DOSAGE MUST BE INDIVIDUALIZED. NADOLOL TABLETS MAY BE ADMINISTERED WITHOUT REGARD TO MEALS. Angina Pectoris The usual initial dose is 40 mg nadolol once daily. Dosage may be gradually increased in 40 to 80 mg increments at 3 to 7 day intervals until optimum clinical response is obtained or there is pronounced slowing of the heart rate. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 160 or 240 mg administered once daily may be needed. The usefulness and safety in angina pectoris of dosage exceeding 240 mg per day have not been established. If treatment is to be discontinued, reduce the dosage gradually over a period of one to two weeks ( see WARNINGS ). Hypertension The usual initial dose is 40 mg nadolol once daily, whether it is used alone or in addition to diuretic therapy. Dosage may be gradually increased in 40 to 80 mg increments until optimum blood pressure reduction is achieved. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 240 or 320 mg administered once daily may be needed. Dosage Adjustment in Renal Failure Absorbed nadolol is excreted principally by the kidneys and, although nonrenal elimination does occur, dosage adjustments are necessary in patients with renal impairment. The following dose intervals are recommended: Creatinine Clearance (mL/min/1.73m 2 ) Dosage Interval (hours) >50 24 31 to 50 24 to 36 10 to 30 24 to 48 <10 40 to 60
