Drug Catalog - Product Detail
MYCOPHENOLATE MOFETIL CP 250MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 67877-0266-01 | ASCEND LABORATORIES | 100 | 250MG | CAPSULE |
PACKAGE FILES




Generic Name
MYCOPHENOLATE MOFETIL
Substance Name
MYCOPHENOLATE MOFETIL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA091249
Description
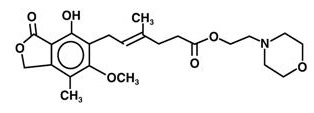
11 DESCRIPTION Mycophenolate mofetil is an antimetabolite immunosuppressant. It is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor. The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate. It has an empirical formula of C 23 H 31 NO 7 , a molecular weight of 433.50, and the following structural formula: Mycophenolate mofetil is a white to off-white crystalline powder. It is slightly soluble in water (43 mcg/mL at pH 7.4); the solubility increases in acidic medium (4.27 mg/mL at pH 3.6). It is freely soluble in acetone, soluble in methanol, and sparingly soluble in ethanol. The apparent partition coefficient in 1-octanol/water (pH 7.4) buffer solution is 238. The pKa values for MMF are 5.6 for the morpholino group and 8.5 for the phenolic group. Mycophenolate mofetil is available for oral administration as capsules containing 250 mg of MMF, tablets containing 500 mg of mycophenolate mofetil. Inactive ingredients in Mycophenolate mofetil capsules USP 250 mg include croscarmellose sodium, pregelatinized starch, povidone (K-90), isopropyl alcohol and magnesium stearate. The capsule shells contain gelatin, sodium lauryl sulphate, FD&C blue 2, red iron oxide, yellow iron oxide and titanium dioxide. The capsule is printed with edible black ink comprised of black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, shellac, strong ammonia solution. Inactive ingredients in mycophenolate mofetil tablets USP 500 mg include croscarmellose sodium, povidone (K-90), isopropyl alcohol, microcrystalline cellulose, magnesium stearate, hypromellose, polyethylene glycol, titanium dioxide, red iron oxide, black iron oxide, and yellow iron oxide. FDA approved dissolution test specifications differ from USP. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 Handling and Disposal Mycophenolate mofetil (MMF) has demonstrated teratogenic effects in humans [see Warnings and Precautions ( 5.1 ) and Use in Specific Populations ( 8.1 )] . Mycophenolate mofetil tablets should not be crushed and mycophenolate mofetil capsules should not be opened or crushed. Wearing disposable gloves is recommended during reconstitution and when wiping the outer surface of the bottle/cap and the table after reconstitution. Avoid inhalation or direct contact with skin or mucous membranes of the powder contained in mycophenolate mofetil capsules, mycophenolate mofetil oral suspension (before or after constitution), or mycophenolate mofetil intravenous (during or after preparation) [see Dosage and Administration ( 2.6 )]. Follow applicable special handling and disposal procedures 1 . 16.2 Mycophenolate Mofetil Capsules 250 mg Capsules Blue-brown, two-piece hard gelatin capsules, printed in black with “266” on the brown body. Sizes Bottle of 100…………………………………………………………NDC 67877-266-01 Bottle of 500…………………………………………………………NDC 67877-266-05 Carton of 30 (3 x 10 unit-dose capsules) …………………………….NDC 67877-266-84 Carton of 100 (10 x 10 unit-dose capsules)………………………….NDC 67877-266-38 Storage Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) 16.3 Mycophenolate Mofetil Tablets 500 mg Tablets Lavender-colored caplet shaped biconvex film coated tablets debossed with “265” on one side and plain on the other. Sizes Bottle of 100…………………………………………………………NDC 67877-225-01 Bottle of 500…………………………………………………………NDC 67877-225-05 Carton of 30 (3 x 10 unit-dose tablets) ………………………………NDC 67877-225-84 Carton of 100 (10 x 10 unit-dose tablets)……………………...….....NDC 67877-225-38 Storage and Dispensing Information: Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature]. Dispense in a tight light-resistant container, as defined in the USP with a child resistant cap.
Indications & Usage
1 INDICATIONS & USAGE Mycophenolate mofetil (MMF) is indicated for the prophylaxis of organ rejection, in adult and pediatric recipients 3 months of age and older of allogeneic kidney [see Clinical Studies ( 14.1 )], heart [see Clinical Studies ( 14.2 )] or liver transplants [see Clinical Studies ( 14.3 )] , in combination with other immunosuppressants. Mycophenolate mofetil is an antimetabolite immunosuppressant indicated for the prophylaxis of organ rejection in adult and pediatric recipients 3 months of age and older of allogeneic kidney, heart or liver transplants, in combination with other immunosuppressants. (1)
Dosage and Administration
2 DOSAGE & ADMINISTRATION ADULTS DOSAGE Kidney Transplant 1g twice daily,orally or intravenously (IV) over no less than 2h (2.2) Heart Transplant 1.5 g twice daily orally or IV, over no less than 2 h (2.3) Liver Transplant 1.5 g twice daily orally or 1 g twice daily IV over no less than 2 h (2.4) PEDIATRICS Kidney Transplant 600mg/m 2 orally twice daily, up to maximum of 2 g daily (2.2) Heart Transplant 600 mg/m 2 orally twice daily (starting dose) up to a maximum of 900 mg/m 2 twice daily (3 g or 15 mL of oral suspension) (2.3) Liver Transplant 600 mg/m 2 orally twice daily (starting dose) up to a maximum of 900 mg/m 2 twice daily (3 g or 15 mL of oral suspension) (2.4) Mycophenolate mofetil intravenous is an alternative when patients cannot tolerate oral medication. Administer within 24 hours following transplantation, until patients can tolerate oral medication, up to 14 days. ( 2.1 ) Reduce or interrupt dosing in the event of neutropenia. ( 2.5 ) See full prescribing information (FPI) for: adjustments for renal impairment and neutropenia ( 2.5 ), preparation of oral suspension and IV solution. (2.6 ) 2.1 Important Administration Instructions Mycophenolate mofetil should not be used without the supervision of a physician with experience in immunosuppressive therapy. Mycophenolate Mofetil Capsules, Tablets and Oral Suspension Mycophenolate mofetil oral dosage forms (capsules, tablets or oral suspension) should not be used interchangeably with mycophenolic acid delayed-release tablets without supervision of a physician with experience in immunosuppressive therapy because the rates of absorption following the administration of mycophenolate mofetil oral dosage forms and mycophenolic acid delayed-release tablets are not equivalent. Mycophenolate mofetil tablets should not be crushed and mycophenolate mofetil capsules should not be opened or crushed. Patients should avoid inhalation or contact of the skin or mucous membranes with the powder contained in mycophenolate mofetil capsules and oral suspension. If such contact occurs, they must wash the area of contact thoroughly with soap and water. In case of ocular contact, rinse eyes with plain water. The initial oral dose of mycophenolate mofetil should be given as soon as possible following kidney, heart or liver transplant. It is recommended that mycophenolate mofetil be administered on an empty stomach. In stable transplant patients, however, mycophenolate mofetil may be administered with food if necessary [see Clinical Pharmacology ( 12.3 )]. Once reconstituted, mycophenolate mofetil for oral suspension must not be mixed with any liquids prior to dose administration. If needed, mycophenolate mofetil for oral suspension can be administered via a nasogastric tube with a minimum size of 8 French (minimum 1.7 mm interior diameter). Patients should be instructed to take a missed dose as soon as they remember, except if it is closer than 2 hours to the next scheduled dose; in this case, they should continue to take mycophenolate mofetil at the usual times. 2.2 Dosage Recommendations for Kidney Transplant Patients Adults The recommended dosage for adult kidney transplant patients is 1 g orally or intravenously infused over no less than 2 hours, twice daily (total daily dose of 2 g). Pediatrics (3 months and older) Pediatric dosing is based on body surface area (BSA). The recommended dosage of mycophenolate mofetil for oral suspension for pediatric kidney transplant patients 3 months and older is 600 mg/m 2 , administered twice daily (maximum total daily dose of 2g or 10 mL of the oral suspension). Pediatric patients with BSA ≥ 1.25 m 2 may be dosed with capsules or tablets as follows: Table 1 Pediatric Kidney Transplant: Dosage Using Capsules or Tablets Body Surface Area Dosage 1.25 m 2 to less than 1.5 m 2 Mycophenolate mofetil capsule 750 mg twice daily (1.5 g total daily dose) Greater than and equal to 1.5 m 2 Mycophenolate mofetil capsules or tablets 1 g twice daily (2 g total daily dose) 2.3 Dosage Recommendations for Heart Transplant Patients Adults The recommended dosage of mycophenolate mofetil for adult heart transplant patients is 1.5 g orally or intravenously infused over no less than 2 hours administered twice daily (total daily dose of 3 g). Pediatrics (3 months and older) The recommended starting dosage of mycophenolate mofetil oral suspension for pediatric heart transplant patients 3 months and older is 600 mg/m 2 , administered twice daily. If well tolerated, the dose can be increased to a maintenance dosage of 900 mg/m 2 twice daily (maximum total daily dose of 3 g or 15 mL of the oral suspension). The dose may be individualized based on clinical assessment. Pediatric patients with BSA ≥1.25 m 2 may be started on therapy with capsules or tablets as follows: Table 2 Pediatric Heart Transplant: Pediatric Starting Dosage Using Capsules or Tablets Body Surface Area Starting Dosage * 1.25 m 2 to less than 1.5 m 2 Mycophenolate mofetil capsule 750 mg twice daily (1.5 g total daily dose) ≥ 1.5 m 2 Mycophenolate mofetil capsules or tablets 1 g twice daily (2 g total daily dose) * Maximum maintenance dose: 3 g total daily. 2.4 Dosage Recommendations for Liver Transplant Patients Adults The recommended dosage of mycophenolate mofetil for adult liver transplant patients is 1.5 g administered orally twice daily (total daily dose of 3 g) or 1 g infused intravenously over no less than 2 hours, twice daily (total daily dose of 2 g). Pediatrics (3 months and older) The recommended starting dosage of mycophenolate mofetil oral suspension for pediatric liver transplant patients 3 months of age and older is 600 mg/m 2 , administered twice daily. If well tolerated, the dose can be increased to a maintenance dosage of 900 mg/m 2 twice daily (maximum total daily dose of 3 g or 15 mL of the oral suspension). The dose may be individualized based on clinical assessment. Pediatric patients with BSA ≥1.25 m 2 may be started on therapy with capsules or tablets as follows: Table 3 Pediatric Liver Transplant: Pediatric Starting Dosage Using Capsules or Tablets Body Surface Area Starting Dosage * 1.25 m 2 to less than 1.5 m 2 mycophenolate mofetil capsule 750 mg twice daily (1.5 g total daily dose) ≥ 1.5 m 2 mycophenolate mofetil capsules or tablets 1 g twice daily (2 g total daily dose) * Maximum maintenance dose: 3 g total daily. 2.5 Dosage Modifications: Patients with Renal Impairment, Neutropenia Renal Impairment No dosage modifications are needed in kidney transplant patients with delayed graft function postoperatively [see Clinical Pharmacology ( 12.3 )] . In kidney transplant patients with severe chronic impairment of the graft (GFR less than 25 mL/min/1.73 m 2 ), do not administer doses of mycophenolate mofetil greater than 1 g twice a day. These patients should be carefully monitored [see Clinical Pharmacology ( 12.3 )]. Neutropenia If neutropenia develops (ANC less than 1.3 x 10 3 /mcL), dosing with mycophenolate mofetil should be interrupted or reduced, appropriate diagnostic tests performed, and the patient managed appropriately [see Warnings and Precautions ( 5.4 ) and Adverse Reactions ( 6.1 )]. 2.6 Preparation Instructions of Oral Suspension and Intravenous for Pharmacists General Preparation Instructions Before Handling the Formulations Mycophenolate mofetil (MMF) has demonstrated teratogenic effects in humans. Follow applicable special handling and disposal procedures 1 [see Warnings and Precautions ( 5.1 ), Adverse Reactions ( 6.2 ), Use in Specific Populations ( 8.1 , 8.3 ), How Supplied/Storage and Handling ( 16.1 )]. Care should be taken to avoid inhalation or direct contact with skin or mucous membranes of the dry powder or the constituted suspension because MMF has demonstrated teratogenic effects in humans . Wearing disposable gloves is recommended during reconstitution and when wiping the outer surface of the bottle/cap and the table surface after reconstitution. If such contact occurs, wash hands thoroughly with soap and water; rinse eyes with water. Alert patients that they and others should also avoid inhalation or contact of the skin or mucous membranes with the oral suspension. Advise them to wash the area thoroughly with soap and water if such contact occurs; if ocular contact occurs, rinse eyes with plain water. Mycophenolate Mofetil For Oral Suspension Mycophenolate mofetil for oral suspension must be reconstituted by the pharmacist prior to dispensing to the patient. Mycophenolate mofetil oral suspension should not be mixed with any other medication. After reconstitution, the oral suspension contains 200 mg/mL MMF. Before proceeding with the reconstitution steps read the general preparation instructions above [see General Preparation Instructions Before Handling the Formulations]. The following are the steps for reconstitution: Tap the closed bottle several times to loosen the powder. Measure 94 mL of water in a graduated cylinder. Add approximately half the total amount of water for reconstitution to the bottle and shake the closed bottle well for about 1 minute. Add the remainder of water and shake the closed bottle well for about 1 minute. Remove the child-resistant cap and push bottle adapter into neck of bottle. Close bottle with child-resistant cap tightly. This will assure the proper seating of the bottle adapter in the bottle and child-resistant status of the cap. Write the date of expiration of the constituted suspension on the bottle label. (The shelf-life of the constituted suspension is 60 days.) Dispense with the “Instruction for Use” and oral dispensers. Alert patients to read the important handling information described in the instructions for use. Store reconstituted suspension at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Storage in a refrigerator at 2°C to 8°C (36°F to 46°F) is acceptable. Do not freeze. Discard any unused portion 60 days after constitution.
