Drug Catalog - Product Detail
MONTELUKAST CHEWABLE TAB. TB 5MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00603-4654-28 | PAR PHARMACEUTICALS | 500 | 5MG | TABLET CHEWABLE |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
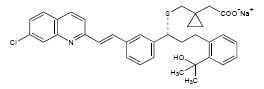
11 DESCRIPTION Montelukast sodium, the active ingredient in montelukast sodium tablets, is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl leukotriene CysLT 1 receptor. Montelukast sodium is described chemically as [ R -( E )]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid, monosodium salt. The empirical formula is C 35 H 35 ClNN a O 3 S, and its molecular weight is 608.18. The structural formula is: Montelukast sodium is a hygroscopic, optically active, white to yellow powder. Montelukast sodium is soluble in water (0.03 g/mL) and freely soluble in methanol, ethanol, DMSO and DMF and practically insoluble in acetonitrile. Each 10‑mg film-coated montelukast sodium tablet contains 10.4 mg montelukast sodium, which is equivalent to 10 mg of montelukast, and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, and silicified microcrystalline cellulose. The film coating consist of: hydroxypropyl cellulose, hypromellose, red ferric oxide, titanium dioxide, and yellow ferric oxide. Each 4-mg and 5-mg chewable montelukast sodium tablet contains 4.2 and 5.2 mg montelukast sodium, respectively, which are equivalent to 4 and 5 mg of montelukast, respectively. Both chewable tablets contain the following inactive ingredients: aspartame, croscarmellose sodium, flavor black cherry, hydroxypropyl cellulose, magnesium stearate, mannitol, red ferric oxide, and silicified microcrystalline cellulose. Structural Formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Montelukast Sodium Chewable Tablets, 4-mg (montelukast), are pink, oval-shaped chewable tablets, debossed with E223 on one side and plain on the other. They are supplied as follows: NDC 0603-4653-16 unit of use high-density polyethylene (HDPE) bottles of 30 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4653-02 unit of use high-density polyethylene (HDPE) bottles of 90 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4653-28 bulk packaging high-density polyethylene (HDPE) bottles of 500 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4653-32 bulk packaging high-density polyethylene (HDPE) bottles of 1,000 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant Montelukast Sodium Chewable Tablets, 5-mg (montelukast), are pink, round-shaped chewable tablets, debossed with E224 on one side and plain on the other. They are supplied as follows: NDC 0603-4654-16 unit of use high-density polyethylene (HDPE) bottles of 30 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4654-02 unit of use high-density polyethylene (HDPE) bottles of 90 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4654-28 bulk packaging high-density polyethylene (HDPE) bottles of 500 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4654-32 bulk packaging high-density polyethylene (HDPE) bottles of 1,000 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant Montelukast Sodium Tablets, 10 mg (montelukast) are round-shaped, beige tablets, debossed with E225 on one side and plain on the other. They are supplied as follows: NDC 0603-4655-16 unit of use high-density polyethylene (HDPE) bottles of 30 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4655-02 unit of use high-density polyethylene (HDPE) bottles of 90 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4655-28 bulk packaging high-density polyethylene (HDPE) bottles of 500 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4655-32 bulk packaging high-density polyethylene (HDPE) bottles of 1,000 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0603-4655-34 bulk packaging high-density polyethylene (HDPE) bottles of 5,000 with a polypropylene cap, an aluminum foil induction seal, and silica gel desiccant Storage Store montelukast 4‑mg chewable tablets, 5‑mg chewable tablets and 10‑mg film-coated tablets at 25°C (77°F), excursions permitted to 15‑30°C (59‑86°F) [see USP Controlled Room Temperature]. Protect from moisture and light. Store in original package.
Indications & Usage
1 INDICATIONS AND USAGE Montelukast sodium tablets are a leukotriene receptor antagonist indicated for: Prophylaxis and chronic treatment of asthma in patients 2 years of age and older ( 1.1 ). Acute prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older ( 1.2 ). Relief of symptoms of allergic rhinitis (AR): seasonal allergic rhinitis (SAR) in patients 2 years of age and older, and perennial allergic rhinitis (PAR) in patients 2 years of age and older ( 1.3 ). 1.1 Asthma Montelukast sodium is indicated for the prophylaxis and chronic treatment of asthma in adults and pediatric patients 2 years of age and older. 1.2 Exercise-Induced Bronchoconstriction (EIB) Montelukast sodium is indicated for prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older. Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp’s montelukast tablet products. However, due to Merck Sharp & Dohme Corp’s marketing exclusivity rights, this drug product is not labeled with that pediatric information. 1.3 Allergic Rhinitis Montelukast sodium is indicated for the relief of symptoms of seasonal allergic rhinitis in patients 2 years of age and older and perennial allergic rhinitis in patients 2 years of age and older.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Administration (by indications): Asthma ( 2.1 ): Once daily in the evening for patients 2 years and older. Acute prevention of EIB ( 2.2 ): One 10 mg tablet at least 2 hours before exercise for patients 15 years of age and older. Seasonal allergic rhinitis ( 2.3 ): Once daily for patients 2 years and older. Perennial allergic rhinitis ( 2.3 ): Once daily for patients 2 years and older. Dosage (by age) (2): 15 years and older: one 10‑mg tablet. 6 to 14 years: one 5‑mg chewable tablet. 2 to 5 years: one 4-mg chewable tablet. Patients with both asthma and allergic rhinitis should take only one dose daily in the evening ( 2.4 ). 2.1 Asthma Montelukast sodium should be taken once daily in the evening. The following doses are recommended: For adults and adolescents 15 years of age and older: one 10‑mg tablet. For pediatric patients 6 to 14 years of age: one 5‑mg chewable tablet. For pediatric patients 2 to 5 years of age: one 4‑mg chewable tablet. Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established. There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing. The pharmacokinetics of montelukast are similar whether dosed in the morning or evening. Efficacy has been demonstrated for asthma when montelukast was administered in the evening without regard to time of food ingestion. 2.2 Exercise-Induced Bronchoconstriction (EIB) in Patients 15 Years of Age and Older For prevention of EIB, a single 10 mg dose of montelukast should be taken at least 2 hours before exercise. An additional dose of montelukast should not be taken within 24 hours of a previous dose. Patients already taking montelukast sodium daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting β‑agonist. Safety and effectiveness in patients younger than 15 years of age have not been established. Daily administration of montelukast sodium for the chronic treatment of asthma has not been established to prevent acute episodes of EIB. Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp’s montelukast tablet products. However, due to Merck Sharp & Dohme Corp’s marketing exclusivity rights, this drug product is not labeled with that pediatric information. 2.3 Allergic Rhinitis For allergic rhinitis, montelukast sodium should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs. The following doses for the treatment of symptoms of seasonal allergic rhinitis are recommended: For adults and adolescents 15 years of age and older: one 10‑mg tablet. For pediatric patients 6 to 14 years of age: one 5‑mg chewable tablet. For pediatric patients 2 to 5 years of age: one 4‑mg chewable tablet. Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established. The following doses for the treatment of symptoms of perennial allergic rhinitis are recommended: For adults and adolescents 15 years of age and older: one 10‑mg tablet. For pediatric patients 6 to 14 years of age: one 5‑mg chewable tablet. For pediatric patients 2 to 5 years of age: one 4‑mg chewable tablet. Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established. 2.4 Asthma and Allergic Rhinitis Patients with both asthma and allergic rhinitis should take only one montelukast sodium dose daily in the evening.
