Drug Catalog - Product Detail
Mometasone Furoate Cream 0.1% 45 GM UoU
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0192-55 | GLENMARK PHARMACEUTICALS | 45 | 0.1% | CREAM |
PACKAGE FILES



Generic Name
MOMETASONE FUROATE
Substance Name
MOMETASONE FUROATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA078541
Description
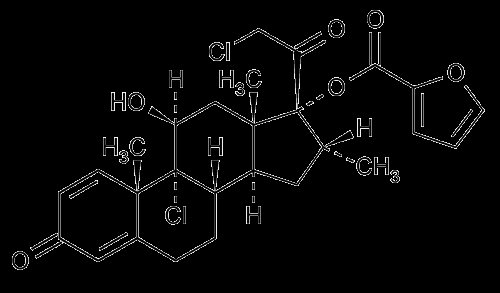
11 DESCRIPTION Mometasone Furoate Cream USP, 0.1% contains mometasone furoate, USP for topical use. Mometasone furoate, USP is a synthetic corticosteroid with anti-inflammatory activity. Chemically, mometasone furoate, USP is 9α,21-dichloro-11β,17-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17-(2-furoate), with the empirical formula C 27 H 30 Cl 2 O 6 , a molecular weight of 521.43 and the following structural formula: Mometasone furoate, USP is a white to off-white powder, soluble in acetone and methylene chloride. Each gram of Mometasone Furoate Cream USP, 0.1% contains: 1 mg mometasone furoate, USP in a cream base of aluminum starch octenyl succinate (Dry-Flo Plus (Pure)), hexylene glycol, phospholipon 90 H, phosphoric acid, purified water, titanium dioxide, white petrolatum, and white wax. mometasone-furoate-structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Mometasone furoate cream USP, 0.1% is a white to off-white, uniform and smooth cream and is supplied in 15 g (NDC 68462-192-17) and 45 g (NDC 68462-192-55) tubes. Store at 25°C (77°F); excursions permitted to 15° to 30°C (59 to86°F) [see USP Controlled Room Temperature]. Avoid excessive heat.
Indications & Usage
1 INDICATIONS AND USAGE Mometasone furoate cream, 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 2 years of age or older. Mometasone furoate cream, 0.1% is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients ≥ 2 years of age. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Apply a thin film of mometasone furoate cream, 0.1% to the affected skin areas once daily. Mometasone furoate cream, 0.1% may be used in pediatric patients 2 years of age or older. Since safety and efficacy of mometasone furoate cream, 0.1% have not been established in pediatric patients below 2 years of age; use in this age group is not recommended [see Warnings and Precautions ( 5.1 ) and Use in Specific Populations ( 8.4 )]. Therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary [see Warnings and Precautions (5.1)]. Do not use mometasone furoate cream, 0.1% with occlusive dressings unless directed by a physician. Do not apply mometasone furoate cream, 0.1% in the diaper area if the patient still requires diapers or plastic pants, as these garments may constitute occlusive dressing. Avoid contact with eyes. Wash hands after each application. Avoid use on the face, groin, or axillae. Mometasone furoate cream, 0.1% is for topical use only. It is not for oral, ophthalmic, or intravaginal use. • Apply a thin film to the affected skin areas once daily. ( 2 ) • Discontinue therapy when control is achieved. ( 2 ) • If no improvement is seen within 2 weeks, reassess diagnosis. ( 2 ) • Do not use with occlusive dressings unless directed by a physician. ( 2 )
