Drug Catalog - Product Detail
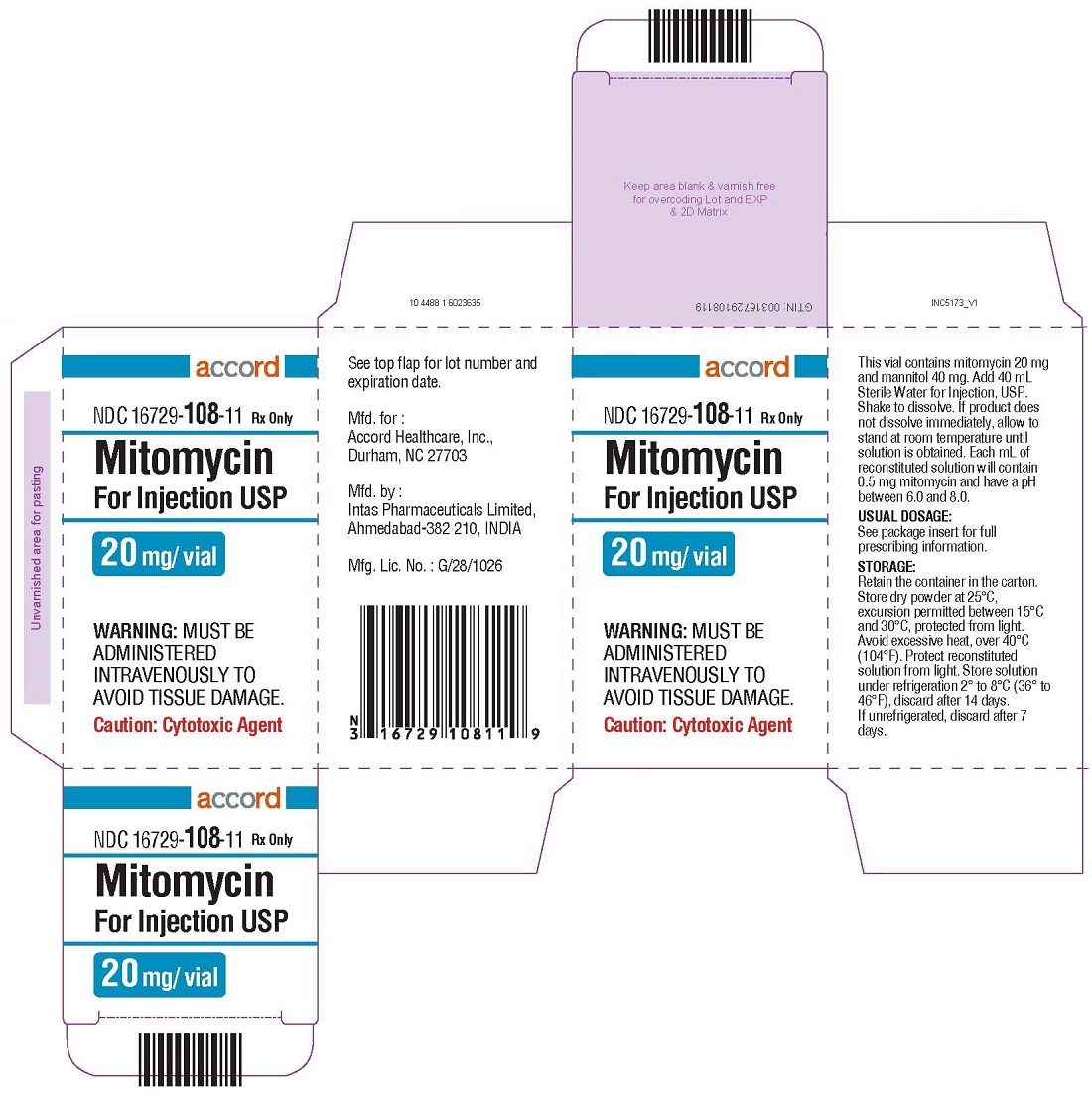
MITOMYCIN FOR INJECTION INJECT. 20MG/50ML 1X50ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16729-0108-11 | ACCORD HEALTHCARE | 1 | 20MG | NA |
PACKAGE FILES


Generic Name
MITOMYCIN
Substance Name
MITOMYCIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAVENOUS
Application Number
ANDA064144
Description
DESCRIPTION Mitomycin (also known as mitomycin and/or mitomycin-C) is an antibiotic isolated from the broth of Streptomyces caespitosus which has been shown to have antitumor activity. The compound is heat stable, has a high melting point, and is freely soluble in organic solvents. Mitomycin for Injection is a sterile dry mixture of mitomycin and mannitol, which when reconstituted with Sterile Water for Injection provides a solution for intravenous administration. Each vial contains either mitomycin 5 mg and mannitol 10 mg, or mitomycin 20 mg and mannitol 40 mg, or mitomycin 40 mg and mannitol 80 mg. Each mL of reconstituted solution will contain 0.5 mg mitomycin and have a pH between 6.0 and 8.0. Mitomycin is a blue-violet crystalline powder with the molecular formula of C 15 H 18 N 4 O 5 , and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane and it has the following structural formula; Structural Formula
How Supplied
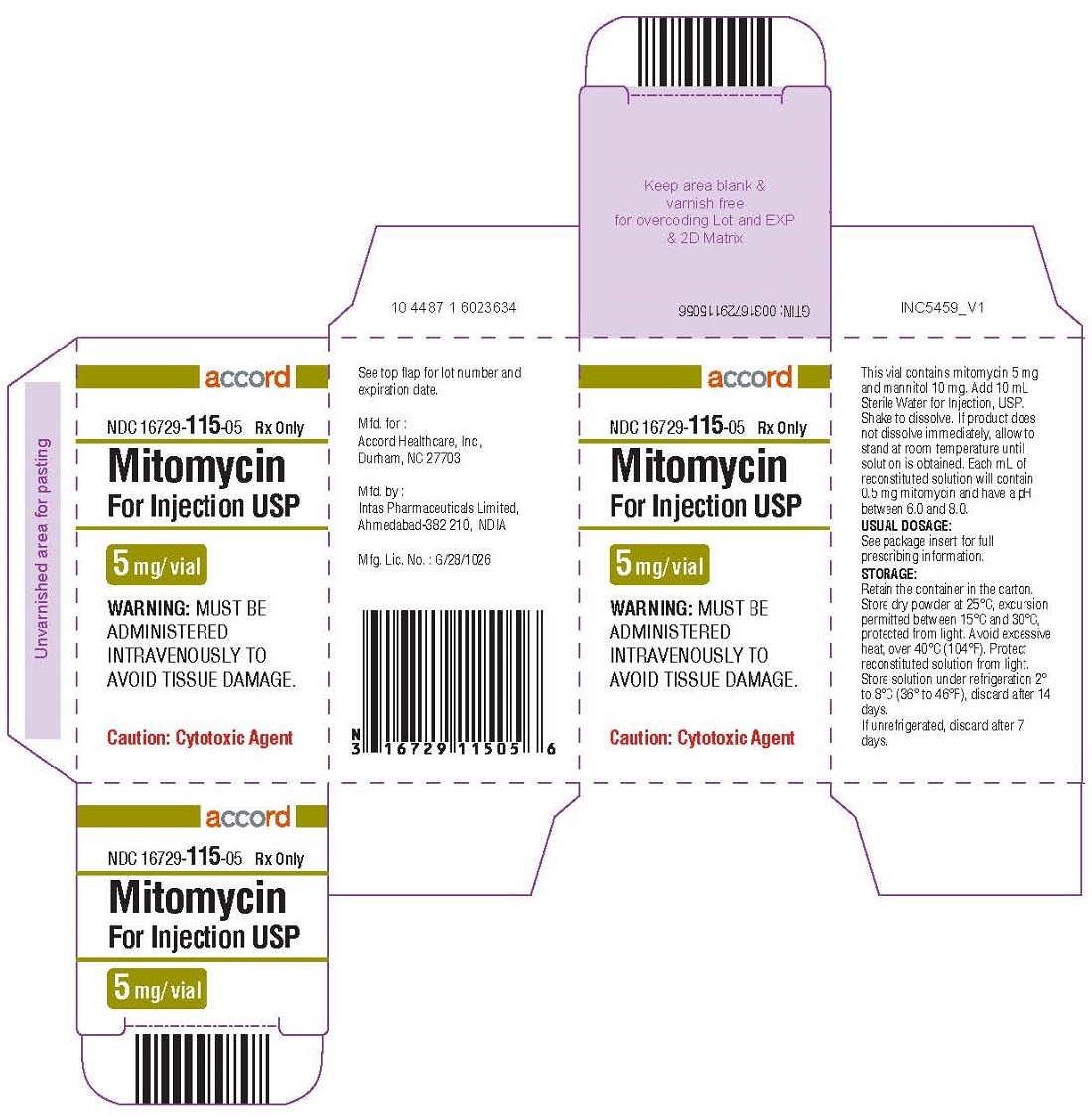
HOW SUPPLIED Mitomycin for Injection USP NDC 16729-115-05—Each amber vial contains 5 mg mitomycin, individually packed in single carton. NDC 16729-108-11—Each amber vial contains 20 mg mitomycin, individually packed in single carton. NDC 16729-116-38—Each amber vial contains 40 mg mitomycin, individually packed in single carton. Storage: Store dry powder at 25°C, excursion permitted between 15°C and 30°C, protected from light. Avoid excessive heat, over 40 °C (104° F). Protect reconstituted solution from light. Store solution under refrigeration 2° to 8 °C (36° to 46°F), discard after 14 days. If unrefrigerated, discard after 7 days.
Indications & Usage
INDICATIONS AND USAGE Mitomycin for Injection is not recommended as single-agent, primary therapy. It has been shown to be useful in the therapy of disseminated adenocarcinoma of the stomach or pancreas in proven combinations with other approved chemotherapeutic agents and as palliative treatment when other modalities have failed. Mitomycin is not recommended to replace appropriate surgery and/or radiotherapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Mitomycin should be given intravenously only, using care to avoid extravasation of the compound. If extravasation occurs, cellulitis, ulceration, and slough may result. Each vial contains either mitomycin 5 mg and mannitol 10 mg, mitomycin, 20 mg and mannitol 40 mg or mitomycin 40 mg and mannitol 80 mg. To administer, add Sterile Water for Injection, 10 mL, 40 mL or 80 mL respectively. Shake to dissolve. If product does not dissolve immediately, allow to stand at room temperature until solution is obtained. After full hematological recovery (see guide to dosage adjustment) from any previous chemotherapy, the following dosage schedule may be used at 6 to 8 week intervals: 20 mg/m 2 intravenously as a single dose via a functioning intravenous catheter. Because of cumulative myelosuppression, patients should be fully reevaluated after each course of mitomycin, and the dose reduced if the patient has experienced any toxicities. Doses greater than 20 mg/m 2 have not been shown to be more effective, and are more toxic than lower doses. The following schedule is suggested as a guide to dosage adjustment: Nadir After Prior Dose Leukocytes/mm 3 Platelets/mm 3 Percentage of Prior Dose To Be Given >4000 >100,000 100% 3000–3999 75,000–99,999 100% 2000–2999 25,000–74,999 70% <2000 <25,000 50% No repeat dosage should be given until leukocyte count has returned to 4000/mm 3 and a platelet count to 100,000/mm 3 . When mitomycin is used in combination with other myelosuppressive agents, the doses should be adjusted accordingly. If the disease continues to progress after two courses of mitomycin, the drug should be stopped since chances of response are minimal. STABILITY Unreconstituted mitomycin stored at room temperature is stable for the lot life indicated on the package. Avoid excessive heat (over 40°C, 104°F). Reconstituted with Sterile Water for Injection to a concentration of 0.5 mg per mL, mitomycin is stable for 14 days refrigerated or 7 days at room temperature. Diluted in various I.V. fluids at room temperature, to a concentration of 20 to 40 micrograms per mL: I.V. Fluid Stability 0.9% Sodium Chloride Injection 12 hours Sodium Lactate Injection 24 hours The combination of mitomycin (5 mg to 15 mg) and heparin (1,000 units to 10,000 units) in 30 mL of 0.9% Sodium Chloride Injection is stable for 48 hours at room temperature. Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published. 1-8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
