Drug Catalog - Product Detail
MINOCYCLINE HCL CAPS 100MG 50CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 57664-0853-85 | SUN PHARMACEUTICALS | 50 | 100MG | NA |
PACKAGE FILES



Generic Name
MINOCYCLINE HYDROCHLORIDE
Substance Name
MINOCYCLINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090867
Description
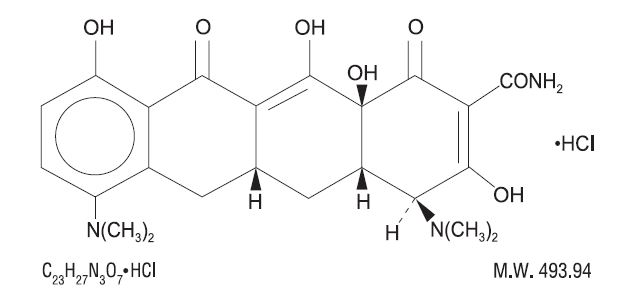
DESCRIPTION Minocycline hydrochloride USP, is a semisynthetic derivative of tetracycline, 4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride. Its structural formula is: C 23 H 27 N 3 O 7 •HCl M.W. 493.94 Minocycline hydrochloride capsules USP for oral administration contain minocycline hydrochloride, USP equivalent to 50 mg, 75 mg, or 100 mg of minocycline and the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, povidone, sodium starch glycolate, colloidal silicon dioxide, magnesium stearate, titanium dioxide, gelatin, D&C Yellow #10 aluminum lake, FD&C Blue #1 aluminum lake, FD&C Blue #2 aluminum lake, FD&C Red #40 aluminum lake, black iron oxide, propylene glycol and shellac. In addition 50 mg capsules contains D&C Red #33 aluminum lake, FD&C Red #3 aluminum lake and FD&C Yellow #6 aluminum lake; 75 mg capsules contains FDA/E172 black iron oxide and 100 mg capsules contains D&C Red #28 aluminum lake, FD&C Blue #1 aluminum lake, FD&C Red #40 aluminum lake and FDA/E172 red iron oxide. structure
How Supplied
HOW SUPPLIED Minocycline hydrochloride Capsules USP, 50 mg are Flesh Opaque/ Flesh Opaque colored hard gelatin capsules, imprinted with ‘851’ on both cap and body in black ink available as follows: Bottles of 100 NDC 57664-851-88 Bottles of 500 NDC 57664-851-13 Minocycline hydrochloride Capsules USP, 75 mg are Gray Opaque/Gray Opaque colored hard gelatin capsules, imprinted with ‘852’ on both cap and body in black ink available as follows: Bottles of 100 NDC 57664-852-88 Bottles of 500 NDC 57664-852-13 Minocycline hydrochloride Capsules USP, 100 mg are Maroon Opaque/ Flesh Opaque colored hard gelatin capsules, imprinted with ‘853’ on both cap and body in black ink available as follows: Bottles of 50 NDC 57664-853-85 Bottles of 500 NDC 57664-853-13 Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required). Protect from light, moisture and excessive heat. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
INDICATIONS AND USAGE Minocycline hydrochloride capsules, USP are indicated in the treatment of the following infections due to susceptible strains of the designated microorganisms: Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox and tick fevers caused by rickettsiae. Respiratory tract infections caused by Mycoplasma pneumoniae . Lymphogranuloma venereum caused by Chlamydia trachomatis . Psittacosis (Ornithosis) due to Chlamydophila psittaci. Trachoma caused by Chlamydia trachomatis , although the infectious agent is not always eliminated, as judged by immunofluorescence. Inclusion conjunctivitis caused by Chlamydia trachomatis . Nongonococcal urethritis, endocervical, or rectal infections in adults caused by Ureaplasma urealyticum or Chlamydia trachomatis . Relapsing fever due to Borrelia recurrentis . Chancroid caused by Haemophilus ducreyi . Plague due to Yersinia pestis . Tularemia due to Francisella tularensis . Cholera caused by Vibrio cholerae. Campylobacter fetus infections caused by Campylobacter fetus. Brucellosis due to Brucella species (in conjunction with streptomycin). Bartonellosis due to Bartonella bacilliformis . Granuloma inguinale caused by Klebsiella granulomatis. Minocycline is indicated for the treatment of infections caused by the following gram-negative microorganisms when bacteriologic testing indicates appropriate susceptibility to the drug: Escherichia coli. Klebsiella aerogenes. Shigella species. Acinetobacter species. Respiratory tract infections caused by Haemophilus influenzae . Respiratory tract and urinary tract infections caused by Klebsiella species. Minocycline hydrochloride capsules, USP are indicated for the treatment of infections caused by the following gram-positive microorganisms when bacteriologic testing indicates appropriate susceptibility to the drug: Upper respiratory tract infections caused by Streptococcus pneumoniae . Skin and skin structure infections caused by Staphylococcus aureus . (NOTE: Minocycline is not the drug of choice in the treatment of any type of staphylococcal infection.) When penicillin is contraindicated, minocycline is an alternative drug in the treatment of the following infections: Uncomplicated urethritis in men due to Neisseria gonorrhoeae and for the treatment of other gonococcal infections. Infections in women caused by Neisseria gonorrhoeae . Syphilis caused by Treponema pallidum subspecies pallidum . Yaws caused by Treponema pallidum subspecies pertenue . Listeriosis due to Listeria monocytogenes . Anthrax due to Bacillus anthracis. Vincent’s infection caused by Fusobacterium fusiforme . Actinomycosis caused by Actinomyces israelii . Infections caused by Clostridium species. In acute intestinal amebiasis, minocycline may be a useful adjunct to amebicides. In severe acne , minocycline may be useful adjunctive therapy. Oral minocycline is indicated in the treatment of asymptomatic carriers of Neisseria meningitidis to eliminate meningococci from the nasopharynx. In order to preserve the usefulness of minocycline in the treatment of asymptomatic meningococcal carriers, diagnostic laboratory procedures, including serotyping and susceptibility testing, should be performed to establish the carrier state and the correct treatment. It is recommended that the prophylactic use of minocycline be reserved for situations in which the risk of meningococcal meningitis is high. Oral minocycline is not indicated for the treatment of meningococcal infection. Although no controlled clinical efficacy studies have been conducted, limited clinical data show that oral minocycline hydrochloride has been used successfully in the treatment of infections caused by Mycobacterium marinum . To reduce the development of drug-resistant bacteria and maintain the effectiveness of minocycline hydrochloride capsules, USP and other antibacterial drugs, minocycline hydrochloride capsules, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
DOSAGE & ADMINISTRATION THE USUAL DOSAGE AND FREQUENCY OF ADMINISTRATION OF MINOCY‑CLINE DIFFERS FROM THAT OF THE OTHER TETRACYCLINES. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE EFFECTS. Minocycline hydrochloride capsules USP may be taken with or without food (see CLINICAL PHARMACOLOGY ). Ingestion of adequate amounts of fluids along with capsule and tablet forms of drugs in the tetracycline-class is recommended to reduce the risk of esophageal irritation and ulceration. The capsules should be swallowed whole. For Pediatric Patients above 8 Years of Age Usual pediatric dose: 4 mg/kg initially followed by 2 mg/kg every 12 hours, not to exceed the usual adult dose. Adults The usual dosage of minocycline hydrochloride capsules USP is 200 mg initially followed by 100 mg every 12 hours. Alternatively, if more frequent doses are preferred, two or four 50 mg capsules may be given initially followed by one 50 mg capsule 4 times daily. Uncomplicated gonococcal infections other than urethritis and anorectal infec‑tions in men: 200 mg initially, followed by 100 mg every 12 hours for a minimum of 4 days, with post-therapy cultures within 2 to 3 days. In the treatment of uncomplicated gonococcal urethritis in men, 100 mg every 12 hours for 5 days is recommended. For the treatment of syphilis, the usual dosage of minocycline hydrochloride should be administered over a period of 10 to 15 days. Close follow-up, including laboratory tests, is recommended. In the treatment of meningococcal carrier state, the recommended dosage is 100 mg every 12 hours for 5 days. Mycobacterium marinum infections: Although optimal doses have not been established, 100 mg every 12 hours for 6 to 8 weeks have been used successfully in a limited number of cases. Uncomplicated urethral, endocervical, or rectal infection in adults caused by Chlamydia trachomatis or Ureaplasma urealyticum : 100 mg orally, every 12 hours for at least 7 days. Ingestion of adequate amounts of fluids along with capsule and tablet forms of drugs in the tetracycline-class is recommended to reduce the risk of esophageal irritation and ulceration. The pharmacokinetics of minocycline in patients with renal impairment (CL CR <80 mL/min) have not been fully characterized. Current data are insufficient to determine if a dosage adjustment is warranted. The total daily dosage should not exceed 200 mg in 24 hours. However, due to the antianabolic effect of tetracyclines, BUN and creatinine should be monitored (See WARNINGS-Antianabolic Action ).
