Drug Catalog - Product Detail
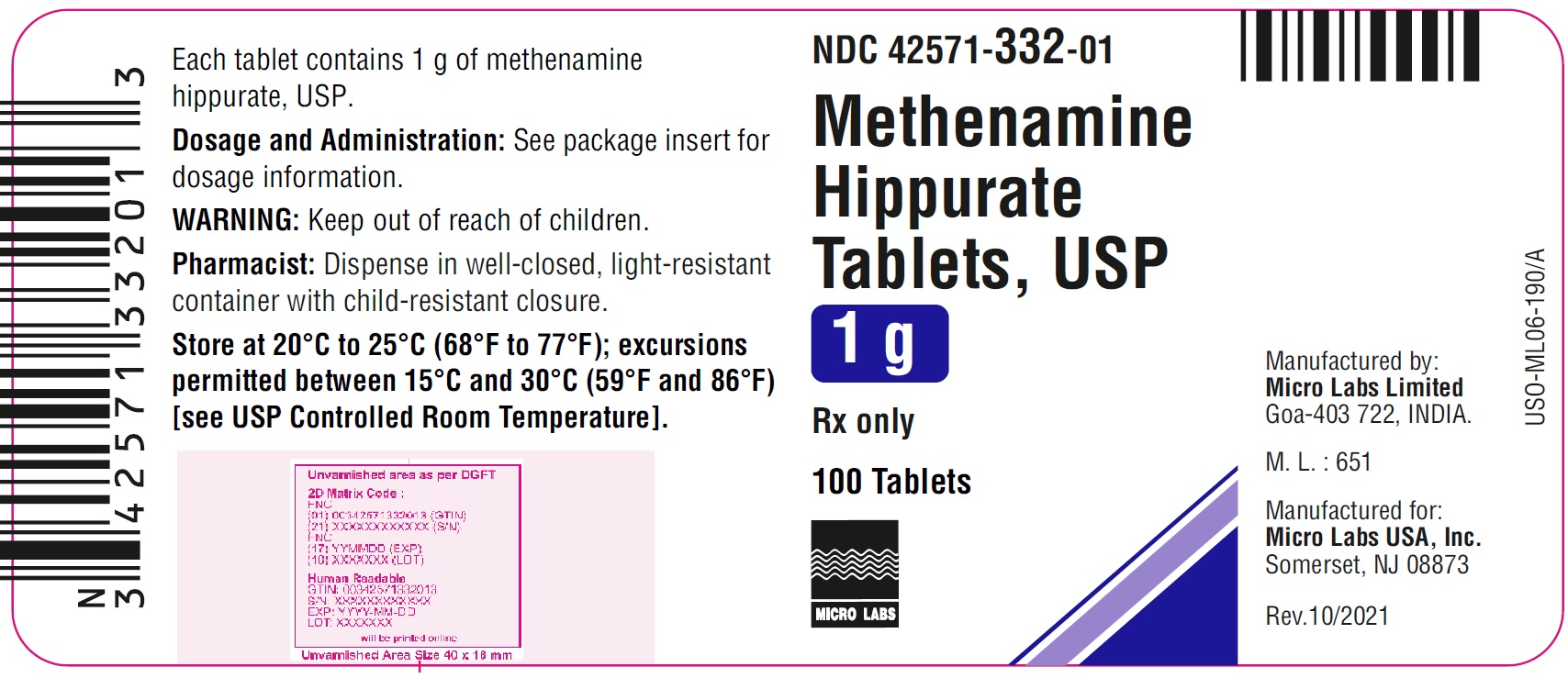
METHENAMINE HIPPURATE 1GM TABS 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 42571-0332-01 | MICRO LABORATORIES | 100 | 1GM | TABLET |
PACKAGE FILES
Generic Name
METHENAMINE HIPPURATE
Substance Name
METHENAMINE HIPPURATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA212172
Description
DESCRIPTION Each white to off-white capsule-shaped tablet contains 1 g Methenamine Hippurate USP which is the Hippuric Acid Salt of Methenamine (hexamethylene tetramine). The tablet also contains inactive ingredients Colloidal silicon dioxide, magnesium stearate and povidone K90.
How Supplied
HOW SUPPLIED White to off white colored, capsule shaped, biconvex tablets, debossed with "H" and "1" on either side of breakline on one side and other side plain with approximate length 20.00 mm, width 8.00 mm and thickness 7.40 mm. Bottles of 100 NDC 42571-332-01 Carton of 80 (8 x10) Unit-dose Tablets NDC 42571-332-23 Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Dispense in well-closed, light-resistant container with child-resistant closure. Manufactured by: Micro Labs Limited Goa-403 722, INDIA. Manufactured for: Micro Labs USA, Inc. Somerset, NJ 08873 Rev.10/2021
Indications & Usage
INDICATIONS Methenamine hippurate tablets USP are indicated for prophylactic or suppressive treatment of frequently recurring urinary tract infections when long-term therapy is considered necessary. This drug should only be used after eradication of the infection by other appropriate antimicrobial agents. To reduce the development of drug-resistant bacteria and maintain the effectiveness of methenamine hippurate and other antibacterial drugs, methenamine hippurate should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION 1 tablet (1 g) twice daily (morning and night) for adults and pediatric patients over 12 years of age. 1/2 to 1 tablet (0.5 to 1 g) twice daily (morning and night) for pediatric patients 6 to 12 years of age. Since the antibacterial activity of methenamine hippurate is greater in acid urine, restriction of alkalinizing foods and medications is desirable. If necessary, as indicated by urinary pH and clinical response, supplemental acidification of the urine should be instituted. The efficacy of therapy should be monitored by repeated urine cultures.
