Drug Catalog - Product Detail
METHAZOLAMIDE TAB 50 MG 100 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62559-0241-01 | ANI PHARMACEUTICALS | 100 | 50MG | TABLET |
PACKAGE FILES



Generic Name
METHAZOLAMIDE
Substance Name
METHAZOLAMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040001
Description
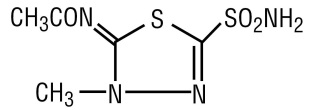
DESCRIPTION Methazolamide, a sulfonamide derivative, is a white crystalline powder, weakly acidic, slightly soluble in water, alcohol and acetone. The chemical name for methazolamide is: N-[5-(aminosulfonyl)-3-methyl-1,3,4-thiadiazo1-2(3H)-ylidene]-acetamide and it has the following structural formula: Molecular Formula: C 5 H 8 N 4 O 3 S 2 Molecular Weight: 236.26 Each tablet, for oral administration, contains 25 mg or 50 mg of methazolamide USP. In addition, each tablet contains the following inactive ingredients: dibasic calcium phosphate dihydrate, glyceryl behenate, povidone, pregelatinized starch, and sodium starch glycolate. chemical structure
How Supplied
HOW SUPPLIED Methazolamide Tablets USP, 25 mg, are white, square, un-scored tablets, debossed “ANI” on one side and “240” on the other side; supplied in bottles of 100 (NDC 62559-240-01). Methazolamide Tablets USP, 50 mg, are white, round tablets, scored on one side and debossed “ANI” and “241” on the other side; supplied in bottles of 100 (NDC 62559-241-01). Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP, with a child-resistant closure (as required). Rx only Manufactured by: ANI Pharmaceuticals, Inc. Baudette, MN 56623 9656 Rev 07/23 ani
Indications & Usage
INDICATIONS AND USAGE Methazolamide Tablets are indicated in the treatment of ocular conditions where lowering intraocular pressure is likely to be of therapeutic benefit, such as chronic open-angle glaucoma, secondary glaucoma, and preoperatively in acute angle-closure glaucoma where lowering the intraocular pressure is desired before surgery.
Dosage and Administration
DOSAGE AND ADMINISTRATION The effective therapeutic dose administered varies from 50 mg to 100 mg two or three times daily. The drug may be used concomitantly with miotic and osmotic agents.
