Drug Catalog - Product Detail
MESALAMINE DR TABS 1.2GM 120CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-2245-22 | ACTAVIS PHARMA | 120 | 1.2GM | TABLET |
PACKAGE FILES

Generic Name
MESALAMINE
Substance Name
MESALAMINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203817
Description
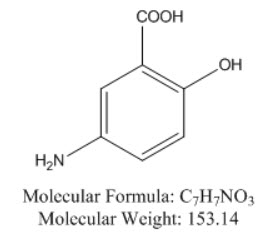
11 DESCRIPTION Each mesalamine delayed-release tablet, USP for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine, USP), an anti-inflammatory agent. Mesalamine, USP also has the chemical name 5-amino-2-hydroxybenzoic acid and its structural formula is: The tablet is coated with a pH-dependent polymer film, which breaks down at or above pH 6.8, normally in the terminal ileum where mesalamine, USP then begins to be released from the tablet core . The inactive ingredients of mesalamine delayed-release tablets, USP are ammonium hydroxide, colloidal silicon dioxide, copovidone, iron oxide black, iron oxide red, iron oxide yellow, magnesium stearate, methacrylic acid and methyl methacrylate copolymer, microcrystalline cellulose, polyethylene glycol 3350, polyvinyl alcohol, povidone, propylene glycol, shellac, sodium starch glycolate (type A), talc, titanium dioxide and triethyl citrate. Meets USP Dissolution Test 3. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Mesalamine delayed-release tablets, USP are available as red-brown, oval shaped, coated tablets containing 1.2 g mesalamine, USP and imprinted with “ WPI 2245 ” in black ink on one side and plain on the other side. NDC 0591-2245-22 (Bottle of 120) Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Mesalamine delayed-release tablets are indicated for the: Induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis. Treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg. Mesalamine is an aminosalicylate indicated for the: Induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis. ( 1 ) Treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Administration Instructions Evaluate renal function prior to initiation of mesalamine delayed-release tablets and periodically while on therapy. Swallow mesalamine delayed-release tablets whole; do not split or crush. Administer mesalamine delayed-release tablets with food [see Clinical Pharmacology ( 12.3 )] . Drink an adequate amount of fluids [see Warnings and Precautions ( 5.8 )] . Adults The recommended dosage for the induction of remission in adult patients with mildly to moderately active ulcerative colitis is 2.4 g to 4.8 g (two to four 1.2 g tablets) taken once daily. The recommended dosage for the maintenance of remission is 2.4 g (two 1.2 g tablets) taken once daily. Pediatric Patients The recommended dosage for treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg who can swallow tablets whole is shown in Table 1: Table 1: Recommended Dosage of Mesalamine Delayed-Release Tablets for the Treatment of Mildly to Moderately Active Ulcerative Colitis in Pediatric Patients Weighing at least 24 kg Weight of Pediatric Patient Once Daily Mesalamine Delayed-Release Tablets Dosage Week 0 to Week 8 After Week 8 24 kg to 35 kg 2.4 g (two 1.2 g tablets) 1.2 g (one 1.2 g tablet) Greater than 35 kg to 50 kg 3.6 g (three 1.2 g tablets) 2.4 g (two 1.2 g tablets) Greater than 50 kg 4.8 g (four 1.2 g tablets) 2.4 g (two 1.2 g tablets) Administration Instructions Evaluate renal function prior to initiation of mesalamine delayed-release tablets and periodically while on therapy. ( 2 , 5.1 ) Swallow mesalamine delayed-release tablets whole; do not split or crush. ( 2 ) Administer mesalamine delayed-release tablets with food. ( 2 ) Drink an adequate amount of fluids. ( 2 , 5.8 ) Recommended Dosage in Adults For induction of remission : 2.4 g to 4.8 g (two to four 1.2 g tablets) once daily. ( 2 ) For maintenance of remission : 2.4 g (two 1.2 g tablets) once daily. ( 2 ) Recommended Dosage in Pediatric Patients The recommended dosage for treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg who can swallow tablets whole is shown below: ( 2 ) Weight of Pediatric Patient Once Daily Mesalamine Delayed-Release Tablets Dosage Week 0 to Week 8 After Week 8 24 kg to 35 kg 2.4 g (two 1.2 g tablets) 1.2 g (one 1.2 g tablet) Greater than 35 kg to 50 kg 3.6 g (three 1.2 g tablets) 2.4 g (two 1.2 g tablets) Greater than 50 kg 4.8 g (four 1.2 g tablets) 2.4 g (two 1.2 g tablets)
