Drug Catalog - Product Detail
MEMANTINE HCL TB 5MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0652-99 | AUROBINDO | NA | NA | NA |
PACKAGE FILES

Generic Name
MEMANTINE HYDROCHLORIDE
Substance Name
MEMANTINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203175
Description
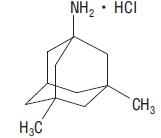
11 DESCRIPTION Memantine hydrochloride is an orally active NMDA receptor antagonist. The chemical name for memantine hydrochloride is 1-amino-3,5-dimethyladamantane hydrochloride with the following structural formula: The molecular formula is C 12 H 21 N•HCl and the molecular weight is 215.76. Memantine hydrochloride USP occurs as a white to off-white, colored powder and is soluble in water. Memantine hydrochloride is available as tablets. Memantine hydrochloride is available for oral administration as modified caplet shaped, film-coated tablets containing 5 mg and 10 mg of memantine hydrochloride USP. The tablets also contain the following inactive ingredients: colloidal silicon dioxide, hydroxy propyl cellulose, microcrystalline cellulose, polyethylene glycol, silicified microcrystalline cellulose, sodium starch glycolate, sodium stearyl fumarate, talc, and titanium dioxide. Memantine Structure
How Supplied
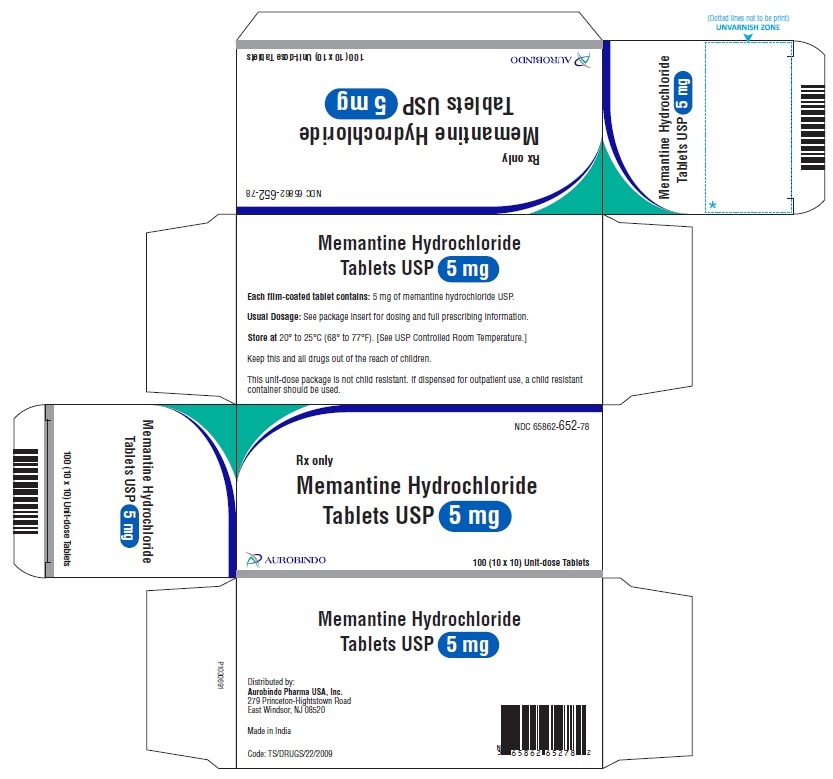
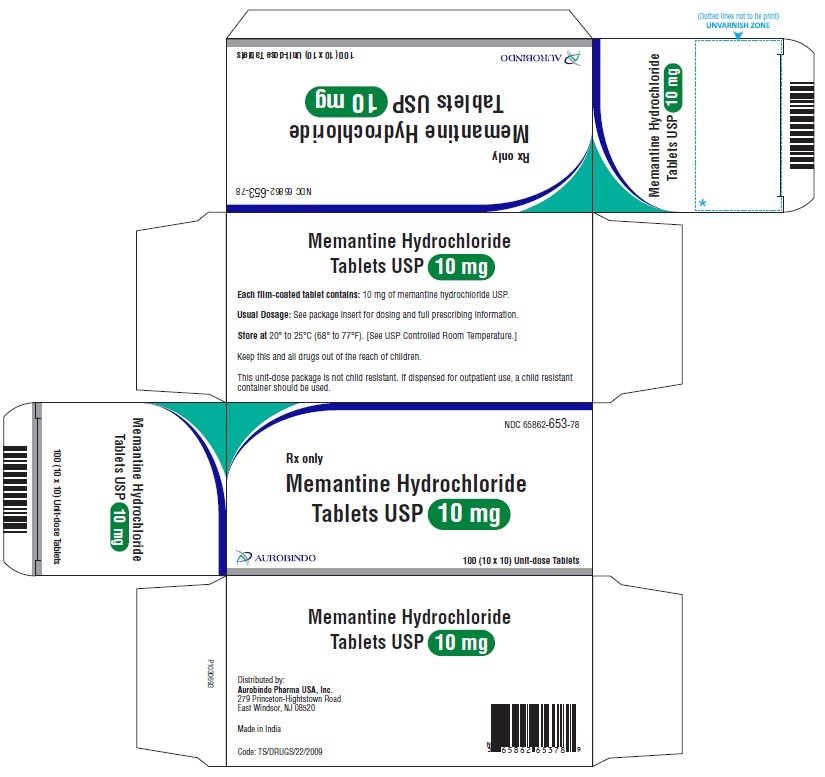
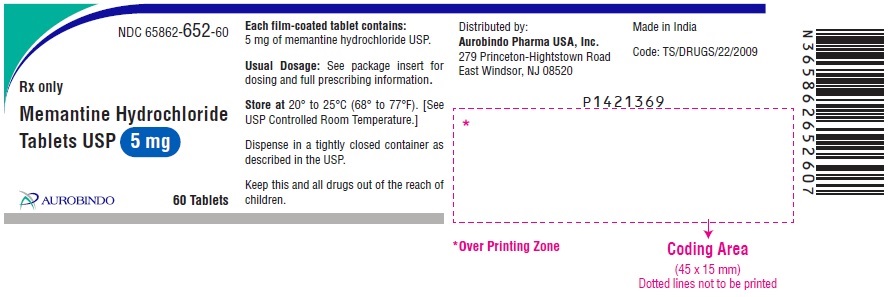
16 HOW SUPPLIED/STORAGE AND HANDLING Memantine Hydrochloride Tablets USP, 5 mg: white to off-white, modified caplet shaped, film-coated tablets debossed with ‘Z’ on one side and ‘01’ on other side Bottles of 60 NDC 65862-652-60 Bottles of 1,000 NDC 65862-652-99 3 x 10 Unit-dose Tablets NDC 65862-652-03 10 x 10 Unit-dose Tablets NDC 65862-652-78 Memantine Hydrochloride Tablets USP, 10 mg: white to off-white, modified caplet shaped, film-coated tablets debossed with ‘Z’ on one side and ‘02’on other side Bottles of 60 NDC 65862-653-60 Bottles of 1,000 NDC 65862-653-99 3 x 10 Unit-dose Tablets NDC 65862-653-03 10 x 10 Unit-dose Tablets NDC 65862-653-78 Store memantine hydrochloride tablets at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Memantine hydrochloride tablets are indicated for the treatment of moderate to severe dementia of the Alzheimer’s type. Memantine hydrochloride tablets are an N-methyl-D-aspartate (NMDA) receptor antagonist indicated for the treatment of moderate to severe dementia of the Alzheimer’s type. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION The recommended starting dose of memantine hydrochloride tablets is 5 mg once daily. The dose should be increased in 5 mg increments to 10 mg/day (5 mg twice daily), 15 mg/day (5 mg and 10 mg as separate doses), and 20 mg/day (10 mg twice daily). The minimum recommended interval between dose increases is one week. The dosage shown to be effective in controlled clinical trials is 20 mg/day. Memantine hydrochloride tablets can be taken with or without food. If a patient misses a single dose of memantine hydrochloride tablets, that patient should not double up on the next dose. The next dose should be taken as scheduled. If a patient fails to take memantine hydrochloride tablets for several days, dosing may need to be resumed at lower doses and retitrated as described above. Specific Populations Renal Impairment A target dose of 5 mg twice daily is recommended in patients with severe renal impairment (creatinine clearance of 5 to 29 mL/min based on the Cockcroft-Gault equation). Hepatic Impairment Memantine hydrochloride tablets should be administered with caution to patients with severe hepatic impairment [see Clinical Pharmacology (12.3) ] . May be taken with or without food. ( 2 ) Initial dose is 5 mg once daily. Increase dose in 5 mg increments to a maintenance dose of 10 mg twice daily. A minimum of 1 week of treatment with the previous dose should be observed before increasing the dose. ( 2 ) Severe renal impairment: recommended dose is 5 mg twice daily. ( 2 )
