Drug Catalog - Product Detail
LORAZEPAM TAB 0.5MG 500CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69315-0904-05 | LEADING PHARMA | 500 | 0.5MG | TABLET |
PACKAGE FILES


Generic Name
LORAZEPAM
Substance Name
LORAZEPAM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078203
Description
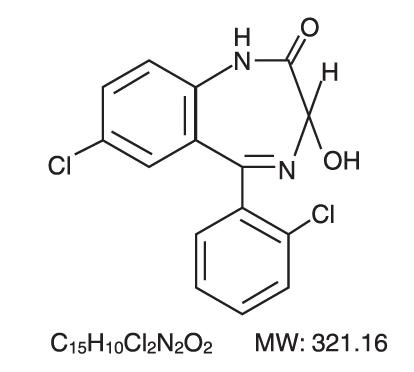
DESCRIPTION Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-( o -chlorophenyl)-1,3-dihydro-3-hydroxy-2 H -1,4-benzodiazepin-2-one: It is a nearly white powder almost insoluble in water. Each Lorazepam tablet, to be taken orally, contains 0.5 mg, 1 mg, or 2 mg of lorazepam. The inactive ingredients present are lactose anhydrous, microcrystalline cellulose, polacrilin potassium and magnesium stearate. Lorazepam structure
How Supplied
HOW SUPPLIED 0.5 mg White color, round, flat face beveled edge compressed tablets, debossed "EP" and "904" on one side, and plain on the other side. NDC# 69315-904-01 Bottles of 100 Tablets. NDC# 69315-904-05 Bottles of 500 Tablets. NDC# 69315-904-10 Bottles of 1000 Tablets. 1 mg White color, round, bisected flat face beveled edge compressed tablets, debossed "EP" above bisect and "905" below bisect on one side, and "1" on the other side. NDC# 69315-905-01 Bottles of 100 Tablets. NDC# 69315-905-05 Bottles of 500 Tablets. NDC# 69315-905-10 Bottles of 1000 Tablets. 2 mg White color, round, bisected flat face beveled edge compressed tablets, debossed "EP" above bisect and "906" below bisect on one side, and "2" on the other side. NDC# 69315-906-01 Bottles of 100 Tablets. NDC# 69315-906-05 Bottles of 500 Tablets. NDC # 69315-906-10 Bottles of 1000 Tablets. BOTTLES: Dispense in a tight, light-resistant container as defined in the USP, using a child-resistant closure. Keep this and all Medications out of the reach of children. Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Indications & Usage
INDICATIONS AND USAGE Lorazepam is indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic. The effectiveness of lorazepam in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Dosage and Administration
DOSAGE AND ADMINISTRATION Lorazepam is administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available. The usual range is 2 to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 to 10 mg/day. For anxiety, most patients require an initial dose of 2 to 3 mg/day given two times a day or three times a day. For insomnia due to anxiety or transient situational stress, a single daily dose of 2 to 4 mg may be given, usually at bedtime. For elderly or debilitated patients, an initial dosage of 1 to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated. The dosage of lorazepam should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
