Drug Catalog - Product Detail
LIDOCAINE TOPICAL PATCH 5%
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00603-1880-16 | ENDO USA | 30 | 5% | TRANSDERMAL SYSTEM |
PACKAGE FILES
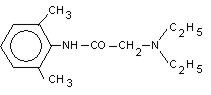


Generic Name
LIDOCAINE
Substance Name
LIDOCAINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
CUTANEOUS
Application Number
NDA020612
Description
DESCRIPTION LIDOCAINE PATCH 5% is comprised of an adhesive material containing 5% lidocaine, which is applied to a non‑woven polyester felt backing and covered with a polyethylene terephthalate (PET) film release liner. The release liner is removed prior to application to the skin. The size of the patch is 10 cm x 14 cm. Lidocaine is chemically designated as acetamide, 2‑(diethylamino)‑N‑(2,6‑ dimethylphenyl), has an octanol: water partition ratio of 43 at pH 7.4, and has the following structure: Each adhesive patch contains 700 mg of lidocaine (50 mg per gram adhesive) in an aqueous base. It also contains the following inactive ingredients: dihydroxyaluminum aminoacetate, disodium edetate, gelatin, glycerin, kaolin, methylparaben, polyacrylic acid, polyvinyl alcohol, propylene glycol, propylparaben, sodium carboxymethylcellulose, sodium polyacrylate, D-sorbitol, tartaric acid, and urea. Chemical Structure
How Supplied
HOW SUPPLIED LIDOCAINE PATCH 5% is available as the following: Carton of 30 patches, packaged into individual child-resistant envelopes NDC 0603-1880-16 Store at 25 o C (77 o F); excursions permitted to 15 o -30 o C (59 o -86 o F). [See USP Controlled Room Temperature]. For more information, call Endo at 1-800-828-9393. Manufactured for: Endo USA Malvern, PA 19355 Revised: April 2024
Indications & Usage
INDICATION AND USAGE LIDOCAINE PATCH 5% is indicated for relief of pain associated with post-herpetic neuralgia. It should be applied only to intact skin .
Dosage and Administration
DOSAGE AND ADMINISTRATION Apply LIDOCAINE PATCH 5% to intact skin to cover the most painful area. Apply the prescribed number of patches (maximum of 3), only once for up to 12 hours within a 24 hour period. Patches may be cut into smaller sizes with scissors prior to removal of the release liner. (See HANDLING AND DISPOSAL ) Clothing may be worn over the area of application. Smaller areas of treatment are recommended in a debilitated patient, or a patient with impaired elimination. If irritation or a burning sensation occurs during application, remove the patch(es) and do not reapply until the irritation subsides. When LIDOCAINE PATCH 5% is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered. LIDOCAINE PATCH 5% may not stick if it gets wet. Avoid contact with water, such as bathing, swimming or showering.
