Drug Catalog - Product Detail
LEVETIRACETAM TB 250MG 120
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0112-16 | LUPIN PHARMACEUTICALS | 120 | 250MG | TABLET |
PACKAGE FILES

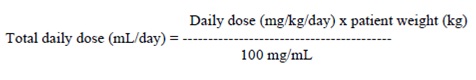
Generic Name
Substance Name
Product Type
Route
Application Number
Description
11. DESCRIPTION Levetiracetam USP is an antiepileptic drug available as 250 mg (blue), 500 mg (yellow), 750 mg (orange), and 1000 mg (white) tablets for oral administration. The chemical name of levetiracetam, a single enantiomer, is (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide, its molecular formula is C H N O and its molecular weight is 170.21. Levetiracetam is chemically unrelated to existing antiepileptic drugs (AEDs). It has the following structural formula: 8 14 2 2 Levetiracetam USP is a white to off-white crystalline powder with a faint odor and a bitter taste. It is very soluble in water (104.0 g/100 mL). It is freely soluble in chloroform (65.3 g/100 mL) and in methanol (53.6 g/100 mL), soluble in ethanol (16.5 g/100 mL), sparingly soluble in acetonitrile (5.7 g/100 mL) and practically insoluble in n-hexane. (Solubility limits are expressed as g/100 mL solvent). Levetiracetam tablets USP contain the labeled amount of levetiracetam. For 250 mg, 500 mg and 750 mg strengths: Inactive ingredients: colloidal silicon dioxide, corn starch, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and additional agents listed below: 250 mg tablets: FD&C Blue No. 2 Aluminum Lake 500 mg tablets: Yellow Iron Oxide 750 mg tablets: FD&C Blue No. 2 Aluminum Lake, FD&C yellow No.6 Aluminum Lake, iron oxide red For 1000 mg strength: Inactive ingredients: colloidal silicon dioxide, corn starch, crospovidone, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, povidone, polyethylene glycol, polyvinyl alcohol, talc and titanium dioxide. Image 2
How Supplied
16. HOW SUPPLIED/STORAGE AND HANDLING NDC:64725-1130-1 in a CONTAINER of 30 TABLET, FILM COATEDS 16.1 How Supplied Levetiracetam tablets USP, 250 mg are blue coloured, oblong-shaped, biconvex, film-coated tablets, debossed with "L" and "U" on either side of the breakline on one side and "X01" on the other side. They are supplied as follows: NDC 68180-112-09 Bottles of 90's NDC 68180-112-16 Bottles of 120's NDC 68180-112-02 Bottles of 500's Levetiracetam tablets USP, 500 mg are yellow coloured, oblong-shaped, biconvex, film-coated tablets, debossed with "L" and "U" on either side of the breakline on one side and "X02" on the other side. They are supplied as follows: NDC 68180-113-09 Bottles of 90's NDC 68180-113-16 Bottles of 120's NDC 68180-113-02 Bottles of 500's Levetiracetam tablets USP, 750 mg are orange coloured, oblong-shaped, biconvex, film-coated tablets, debossed with "L" and "U" on either side of the breakline on one side and "X03" on the other side. They are supplied as follows: NDC 68180-114-09 Bottles of 90's NDC 68180-114-16 Bottles of 120's NDC 68180-114-02 Bottles of 500's Levetiracetam tablets USP, 1000 mg are white to off-white coloured, oblong-shaped, biconvex, film-coated tablets, debossed with "L" and "U" on either side of the breakline on one side and "X04" on the other side. They are supplied as follows: NDC 68180-115-07 Bottles of 60's NDC 68180-115-02 Bottles of 500's 16.2 Storage Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container with child-resistant closure along with medication guide provided separately. Pharmacist:
Indications & Usage
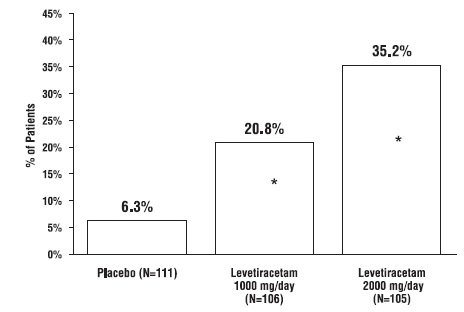
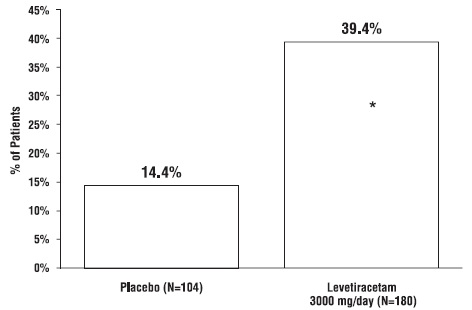
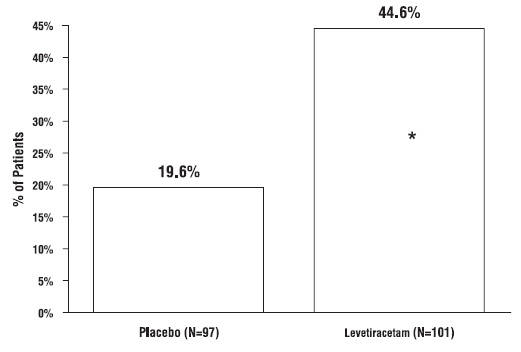
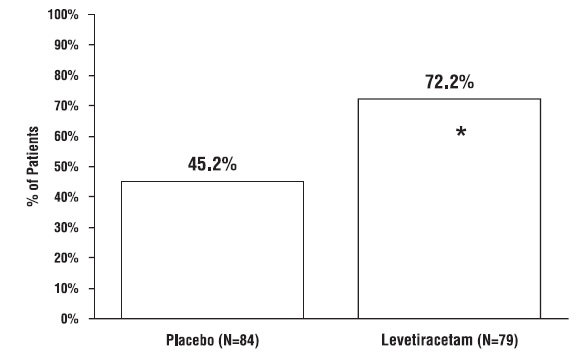
1. INDICATIONS AND USAGE Levetiracetam tablets USP are antiepileptic drugs indicated for adjunctive therapy in the treatment of: Partial onset seizures in patients 4 years of age and older with epilepsy ( ) 1.1 Myoclonic seizures in patients 12 years of age and older with juvenile myoclonic epilepsy ( ) 1.2 Primary generalized tonic-clonic seizures in patients 6 years of age and older with idiopathic generalized epilepsy ( ) 1.3 1.1 Partial Onset Seizures Levetiracetam tablets USP are indicated as adjunctive therapy in the treatment of partial onset seizures in adults and children 4 years of age and older with epilepsy. Information describing the use of levetiracetam in pediatric patients less than 4 years of age as adjunctive therapy in the treatment of partial onset seizures is approved for UCB, Inc.'s levetiracetam tablets. However, due to UCB, Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information. 1.2 Myoclonic Seizures in Patients with Juvenile Myoclonic Epilepsy Levetiracetam tablets USP are indicated as adjunctive therapy in the treatment of myoclonic seizures in adults and adolescents 12 years of age and older with juvenile myoclonic epilepsy. 1.3 Primary Generalized Tonic-Clonic Seizures Levetiracetam tablets USP are indicated as adjunctive therapy in the treatment of primary generalized tonic-clonic seizures in adults and children 6 years of age and older with idiopathic generalized epilepsy.
Dosage and Administration
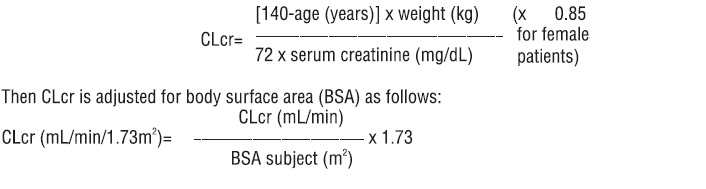
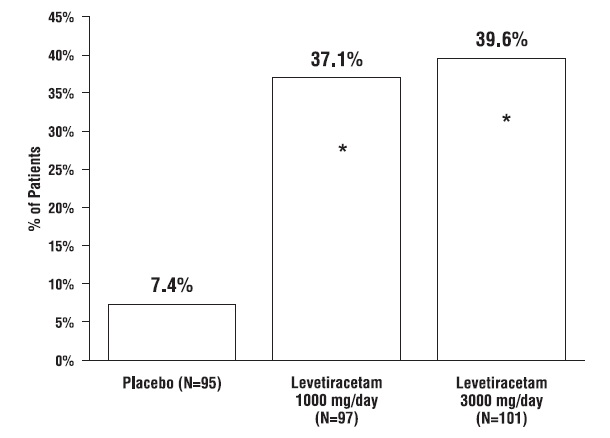
2. DOSAGE AND ADMINISTRATION Use the oral solution for pediatric patients with body weight ≤ 20 kg ( ). 2.1 For pediatric patients, use weight-based dosing for the oral solution with a calibrated measuring device (not a household teaspoon or tablespoon) ( ) 2.1 Partial Onset Seizures : 10 mg/kg twice daily, increase in increments of 10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kg twice daily ( ) 4 Years to < 16 Years 2.2 : 500 mg twice daily, increase as needed and tolerated in increments of 500 mg twice daily every 2 weeks to a maximum recommended dose of 1500 mg twice daily ( ) Adults 16 Years and Older 2.2 Myoclonic Seizures in Adults and Pediatric Patients 12 Years and Older 500 mg twice daily, increase by 500 mg twice daily every 2 weeks to recommended dose of 1500 mg twice daily ( ) 2.3 Primary Generalized Tonic-Clonic Seizures : 10 mg/kg twice daily, increase in increments of 10 mg/kg twice daily every 2 weeks to recommended dose of 30 mg/kg twice daily ( ) 6 Years To < 16 Years 2.4 : 500 mg twice daily, increase by 500 mg twice daily every 2 weeks to recommended dose of 1500 mg twice daily ( ) Adults 16 Years and Older 2.4 Adult Patients with Impaired Renal Function Dose adjustment is recommended, based on the patient's estimated creatinine clearance ( , ) 2.5 8.6 2.1 Important Administration Instructions Levetiracetam tablets are given orally with or without food. The levetiracetam dosing regimen depends on the indication, age group, dosage form (tablets or oral solution), and renal function. Prescribe the oral solution for pediatric patients with body weight ≤ 20 kg. Prescribe the oral solution or tablets for pediatric patients with body weight above 20 kg. Levetiracetam tablets should be swallowed whole. Levetiracetam tablets should not be chewed or crushed. 2.2 Partial Onset Seizures Adults 16 Years and Older In clinical trials, daily doses of 1000 mg, 2000 mg, and 3000 mg, given as twice-daily dosing were shown to be effective. Although in some studies there was a tendency toward greater response with higher dose , a consistent increase in response with increased dose has not been shown. [see CLINICAL STUDIES ( )] 14.1 Treatment should be initiated with a daily dose of 1000 mg/day, given as twice-daily dosing (500 mg twice daily). Additional dosing increments may be given (1000 mg/day additional every 2 weeks) to a maximum recommended daily dose of 3000 mg. Doses greater than 3000 mg/day have been used in open-label studies for periods of 6 months and longer. There is no evidence that doses greater than 3000 mg/day confer additional benefit. Pediatric Patients Dosing information in pediatric patients less than 4 years of age as adjunctive therapy in the treatment of partial onset seizures is approved for UCB, Inc.'s levetiracetam tablets. However, due to UCB, Inc.'s marketing exclusivity rights, this drug product is not labeled with that pediatric information. 4 Years to < 16 Years: Treatment should be initiated with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). The daily dose should be increased every 2 weeks by increments of 20 mg/kg to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). If a patient cannot tolerate a daily dose of 60 mg/kg, the daily dose may be reduced. In the clinical efficacy trial, the mean daily dose was 44 mg/kg. The maximum daily dose was 3000 mg/day. For levetiracetam tablet dosing in pediatric patients weighing 20 to 40 kg, treatment should be initiated with a daily dose of 500 mg given as twice daily dosing (250 mg twice daily). The daily dose should be increased every 2 weeks by increments of 500 mg to a maximum recommended daily dose of 1500 mg (750 mg twice daily). For levetiracetam tablet dosing in pediatric patients weighing more than 40 kg, treatment should be initiated with a daily dose of 1000 mg/day given as twice daily dosing (500 mg twice daily). The daily dose should be increased every 2 weeks by increments of 1000 mg/day to a maximum recommended daily dose of 3000 mg (1500 mg twice daily). Levetiracetam Oral Solution Weight-Based Dosing Calculation For Pediatric Patients The following calculation should be used to determine the appropriate daily dose of oral solution for pediatric patients: 2.3 Myoclonic Seizures in Patients 12 Years of Age and Older with Juvenile Myoclonic Epilepsy Treatment should be initiated with a dose of 1000 mg/day, given as twice-daily dosing (500 mg twice daily). Dosage should be increased by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg. The effectiveness of doses lower than 3000 mg/day has not been studied. 2.4 Primary Generalized Tonic-Clonic Seizures Adults 16 Years and Older Treatment should be initiated with a dose of 1000 mg/day, given as twice-daily dosing (500 mg twice daily). Dosage should be increased by 1000 mg/day every 2 weeks to the recommended daily dose of 3000 mg. The effectiveness of doses lower than 3000 mg/day has not been adequately studied. Pediatric Patients Ages 6 to <16 Years Treatment should be initiated with a daily dose of 20 mg/kg in 2 divided doses (10 mg/kg twice daily). The daily dose should be increased every 2 weeks by increments of 20 mg/kg to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). The effectiveness of doses lower than 60 mg/kg/day has not been adequately studied. Patients with body weight ≤ 20 kg should be dosed with oral solution. Patients with body weight above 20 kg can be dosed with either tablets or oral solution . Only whole tablets should be administered. [see DOSAGE AND ADMINISTRATION ( )] 2.1 2.5 Adult Patients with Impaired Renal Function Levetiracetam tablets dosing must be individualized according to the patient's renal function status. Recommended doses and adjustment for dose for adults are shown in Table 1. In order to calculate the dose recommended for patients with renal impairment, creatinine clearance adjusted for body surface area must be calculated. To do this an estimate of the patient's creatinine clearance (CL ) in mL/min must first be calculated using the following formula: cr Table 1: Dosing Adjustment Regimen for Adult Patients with Impaired Renal Function Group Creatinine Clearance ( mL / min / 1 . 73 m 2 ) Dosage ( mg ) Frequency Normal >80 500 to 1,500 Every 12 hours Mild 50 to 80 500 to 1,000 Every 12 hours Moderate 30 to 50 250 to 750 Every 12 hours Severe <30 250 to 500 Every 12 hours ESRD patients using dialysis ----- 500 to 1000 Following dialysis, a 250 to 500 mg supplemental dose is recommended. Every 24 hours Image 0 Image 1
