Drug Catalog - Product Detail
LEVETIRACETAM ER TB 750MG 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43547-0346-06 | SOLCO HEALTHCARE | 60 | 750MG | TABLET |
PACKAGE FILES
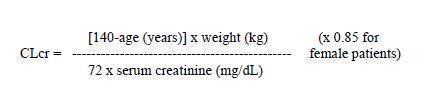




Generic Name
LEVETIRACETAM
Substance Name
LEVETIRACETAM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203468
Description
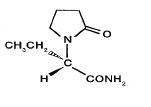
11 DESCRIPTION Levetiracetam extended-release tablets, USP are an antiepileptic drug available as 500 mg and 750 mg (white) extended-release tablets for oral administration. The chemical name of levetiracetam, a single enantiomer, is (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide, its molecular formula is C 8 H 14 N 2 O 2 and its molecular weight is 170.21. Levetiracetam is chemically unrelated to existing antiepileptic drugs (AEDs). It has the following structural formula: Levetiracetam is a white to off-white crystalline powder with a faint odor and a bitter taste. It is very soluble in water (104.0 g/100 mL). It is freely soluble in chloroform (65.3 g/100 mL) and in methanol (53.6 g/100 mL), soluble in ethanol (16.5 g/100 mL), sparingly soluble in acetonitrile (5.7 g/100 mL) and practically insoluble in n-hexane. (Solubility limits are expressed as g/100 mL solvent.) Levetiracetam extended-release tablets, USP contain the labeled amount of levetiracetam, USP. Inactive ingredients: colloidal silicon dioxide, hydroxypropylcellulose, hypromellose, macrogol/peg 3350, magnesium stearate, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide. FDA approved dissolution test specifications differ from USP. The medication is combined with a drug release controlling polymer that provides a drug release at a controlled rate. The biologically inert components of the tablet may occasionally remain intact during GI transit and will be eliminated in the feces as a soft, hydrated mass. Levetiracretam structural formula
How Supplied
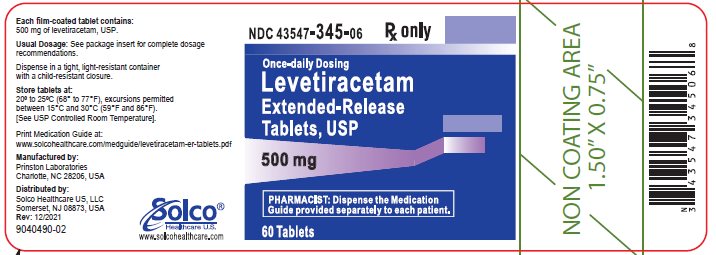
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Levetiracetam extended-release 500 mg tablets, USP are white, oval, biconvex film-coated extended-release tablets debossed with "HH" on one side and “172” on the other side. They are supplied in white HDPE bottles as follows: NDC 43547-345-06: bottles of 60 NDC 43547-345-50: bottles of 500 Levetiracetam extended-release 750 mg tablets, USP are white, oval, biconvex film-coated extended-release tablets debossed with "HH" on one side and “173” on the other side. They are supplied in white HDPE bottles as follows: NDC 43547-346-06: bottles of 60 NDC 43547-346-50: bottles of 500 16.2 Storage Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Indications & Usage
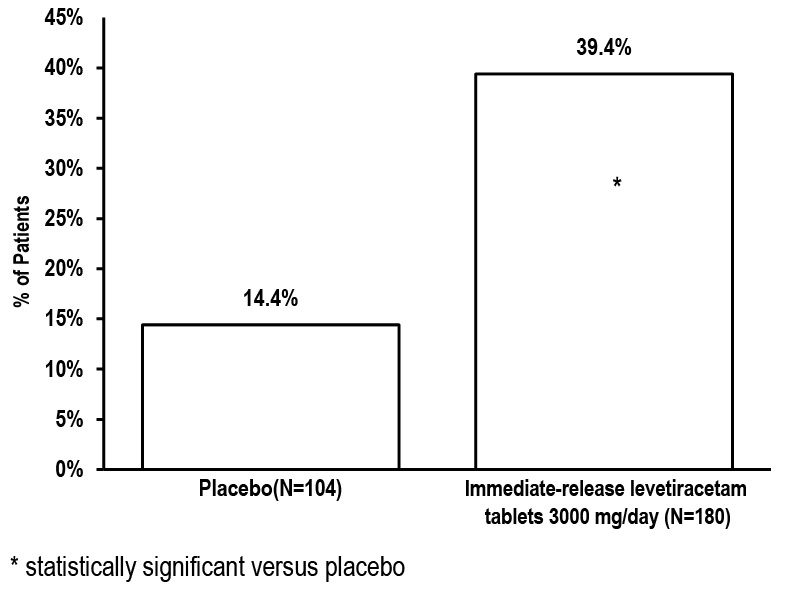
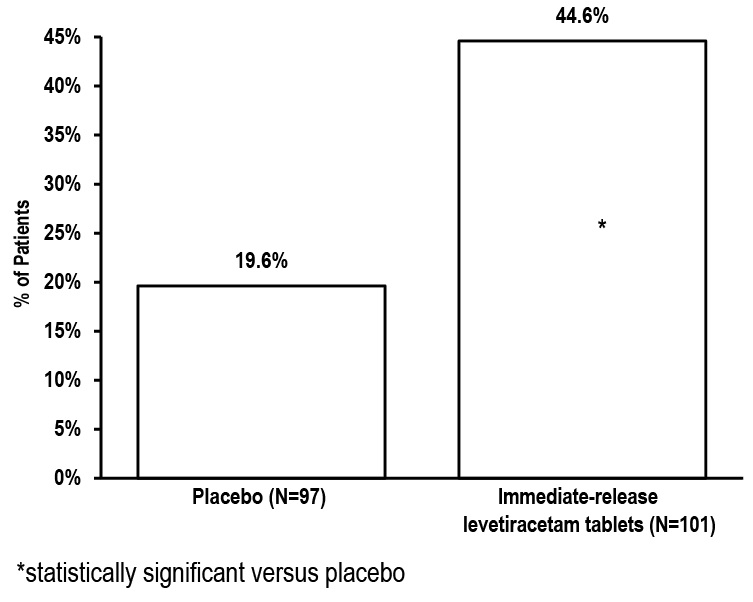
1 INDICATIONS AND USAGE Levetiracetam extended-release tablets are indicated for the treatment of partial-onset seizures in patients 12 years of age and older. Levetiracetam extended-release tablets are indicated for the treatment of partial-onset seizures in patients 12 years of age and older ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Initiate treatment with a dose of 1,000 mg once daily; increase by 1,000 mg every 2 weeks to a maximum recommended dose of 3,000 mg once daily ( 2 ) See full prescribing information for use in patients with impaired renal function ( 2.1 ) 2.1 Recommended Dosing For adults and adolescent patients, the recommended dosing for monotherapy and adjunctive therapy is the same; as outlined below. Adults and Adolescents 12 Years of Age and Older Weighing 50 kg or More Initiate treatment with a dose of 1,000 mg once daily. The once daily dosage may be adjusted in increments of 1,000 mg every 2 weeks to a maximum recommended daily dose of 3,000 mg/day once daily. Administration Levetiracetam extended-release tablets are administered once daily. Levetiracetam extended-release tablets should be swallowed whole. The tablets should not be chewed, broken, or crushed. 2.2 Dosage Adjustments in Adult Patients with Renal Impairment Levetiracetam extended-release tablets dosing must be individualized according to the patient's renal function status. Recommended dosage adjustments for adults are shown in Table 1. In order to calculate the dose recommended for patients with renal impairment, creatinine clearance adjusted for body surface area must be calculated. To do this, an estimate of the patient's creatinine clearance (CLcr) in mL/min must first be calculated using the following formula: Table 1: Dosage Adjustment Regimen for Adult Patients with Renal Impairment Group Creatinine Clearance (mL/min/1.73m 2 ) Dosage (mg) Frequency Normal > 80 1,000 to 3,000 Every 24 hours Mild 50 – 80 1,000 to 2,000 Every 24 hours Moderate 30 – 50 500 to 1,500 Every 24 hours Severe < 30 500 to 1,000 Every 24 hours 1 2 2.3 Discontinuation of Levetiracetam Extended-Release Tablets Avoid abrupt withdrawal from levetiracetam extended-release tablets in order to reduce the risk of increased seizure frequency and status epilepticus [see Warnings and Precautions ( 5.8 )].
