Drug Catalog - Product Detail
LEVALBUTEROL HCL INJ SOL 0.63MG/3ML 25 X 3ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-9931-78 | AMNEAL PHARMACEUTICALS | 3 | 0.63MG/3ML | NEBULIZER SOLUTION |
PACKAGE FILES

Generic Name
LEVALBUTEROL HYDROCHLORIDE
Substance Name
LEVALBUTEROL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
RESPIRATORY (INHALATION)
Application Number
ANDA203653
Description
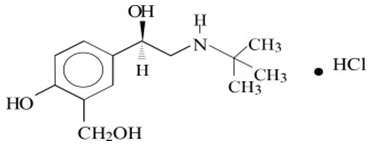
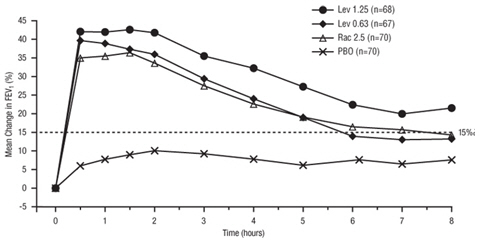
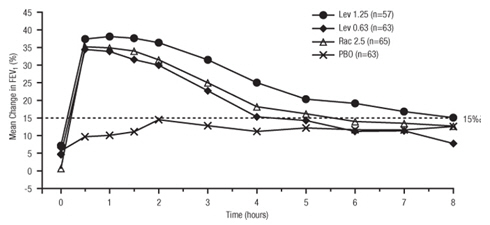
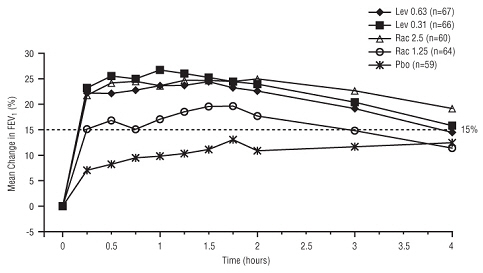
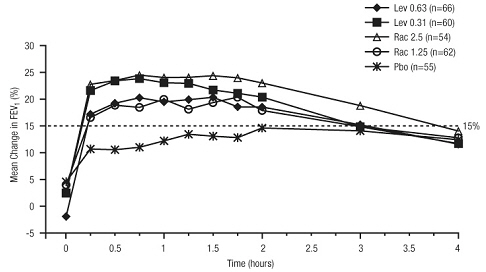
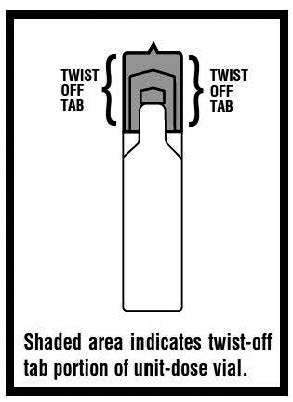
11 DESCRIPTION Levalbuterol Inhalation Solution, USP is a sterile, clear, colorless, preservative-free solution of the hydrochloride salt of levalbuterol, the (R)-enantiomer of the drug substance racemic albuterol. Levalbuterol HCl is a relatively selective beta 2 -adrenergic receptor agonist [see Clinical Pharmacology (12) ]. The chemical name for levalbuterol HCl is (R)-α 1 -[[(1,1-dimethylethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol hydrochloride, and its established chemical structure is as follows: The molecular weight of levalbuterol HCl is 275.8, and its empirical formula is C 13 H 21 NO 3 ∙HCl. It is a white to off-white, crystalline solid, with a melting point of approximately 187°C and solubility of approximately 180 mg/mL in water. Levalbuterol HCl is the USAN modified name for (R)-albuterol HCl in the United States. Levalbuterol Inhalation Solution, USP is supplied in unit-dose vials and requires no dilution before administration by nebulization. Each 3 mL unit-dose vial contains 0.31 mg/3 mL (0.0103%) of levalbuterol (as 0.36 mg/3 mL of levalbuterol HCl) or 0.63 mg/3 mL (0.021%) of levalbuterol (as 0.73 mg/3 mL of levalbuterol HCl) or 1.25 mg/3 mL (0.042%) of levalbuterol (as 1.44 mg/3 mL of levalbuterol HCl), sodium chloride to adjust tonicity, edetate disodium (EDTA) as a stabilizer for the active pharmaceutical ingredient, and sulfuric acid to adjust the pH to 4.0 (3.3 to 4.5). Chemical Structure
How Supplied
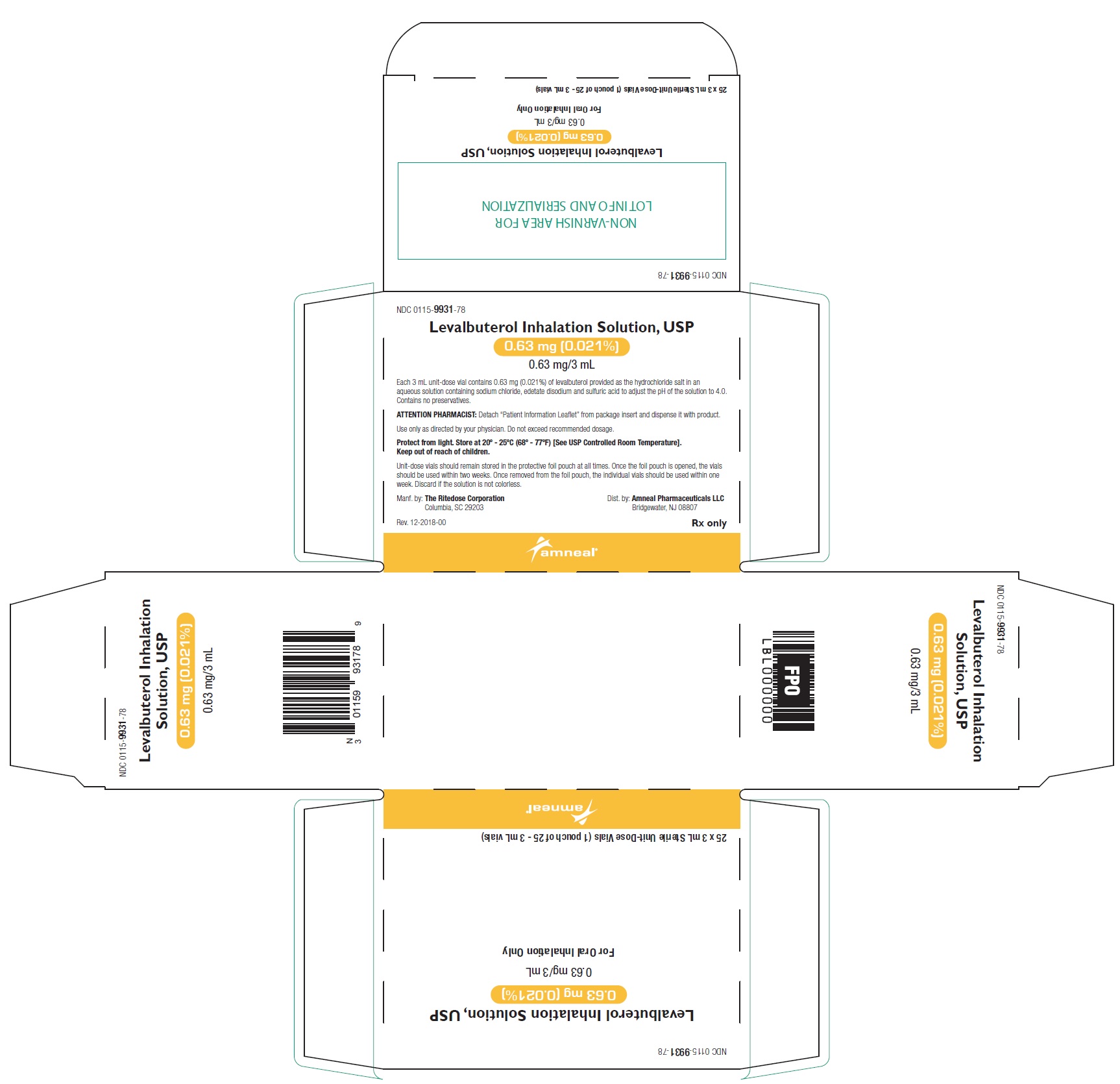
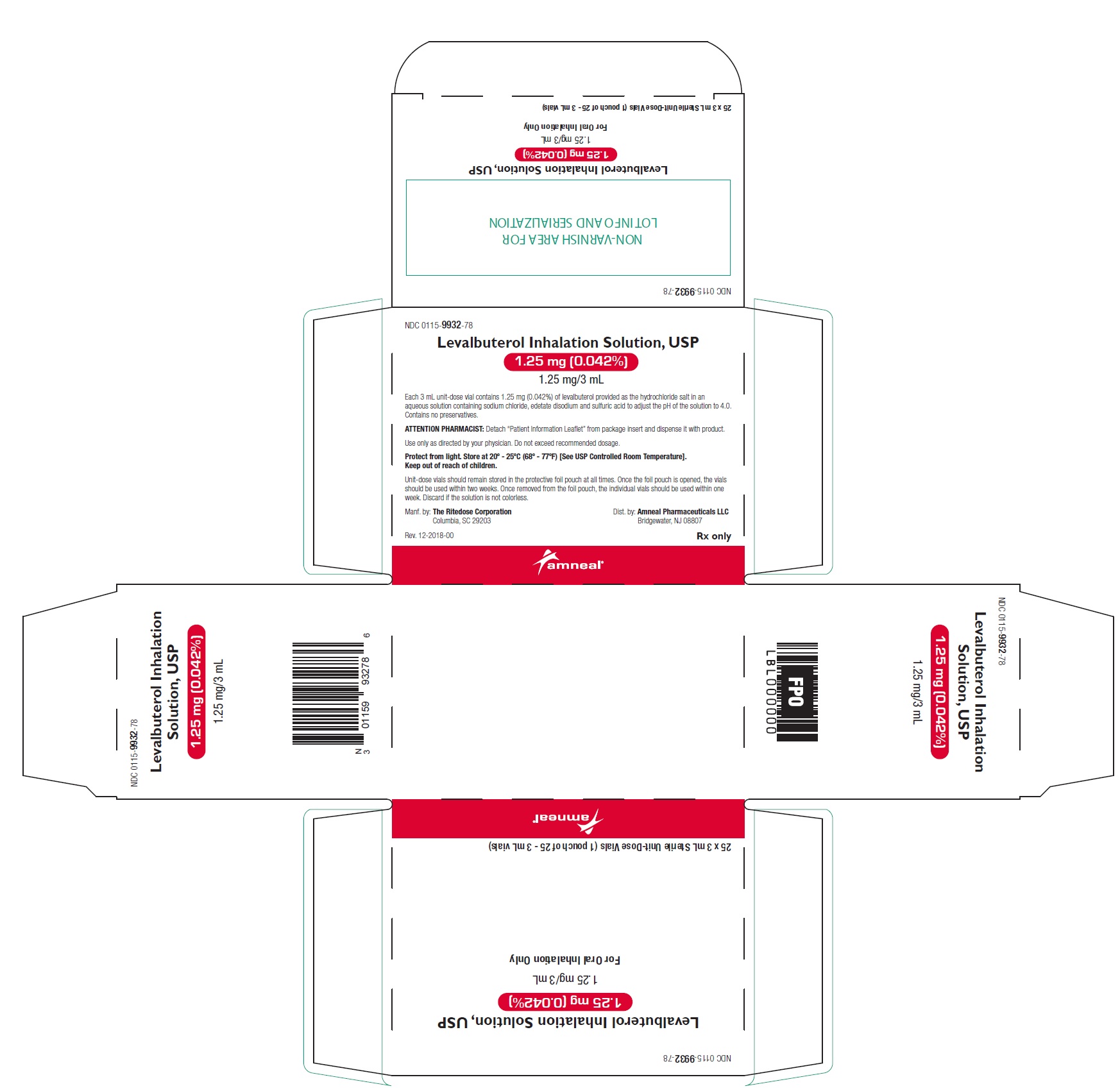
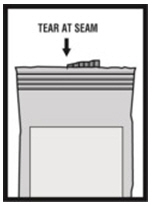
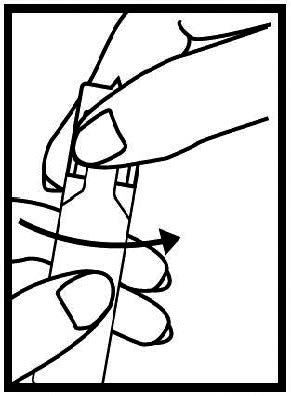
16 HOW SUPPLIED/STORAGE AND HANDLING Levalbuterol Inhalation Solution, USP is supplied in 3 mL unit-dose, low-density polyethylene (LDPE) vials as a clear, colorless, sterile, preservative-free, aqueous solution, in three different strengths of levalbuterol (0.31 mg, 0.63 mg, 1.25 mg). Levalbuterol Inhalation Solution, USP, 0.31 mg/3 mL ( foil pouch label color green ) contains 0.31 mg/3 mL (0.0103%) of levalbuterol (as 0.36 mg/3 mL of levalbuterol HCl) and is available in cartons as listed below. NDC 0115-9930-78 25 vials per carton / 25 vials per foil pouch Levalbuterol Inhalation Solution, USP, 0.63 mg/3 mL ( foil pouch label color yellow ) contains 0.63 mg/3 mL (0.021%) of levalbuterol (as 0.73 mg/3 mL of levalbuterol HCl) and is available in cartons as listed below. NDC 0115-9931-78 25 vials per carton / 25 vials per foil pouch Levalbuterol Inhalation Solution, USP, 1.25 mg/3 mL ( foil pouch label color red ) contains 1.25 mg/3 mL (0.042%) of levalbuterol (as 1.44 mg/3 mL of levalbuterol HCl) and is available in cartons as listed below. NDC 0115-9932-78 25 vials per carton / 25 vials per foil pouch Store Levalbuterol Inhalation Solution, USP in the protective foil pouch at 20°- 25°C (68°- 77°F) [see USP Controlled Room Temperature]. Protect from light and excessive heat. Keep unopened vials in the foil pouch. Once the foil pouch is opened, the vials should be used within 2 weeks. Vials removed from the pouch, if not used immediately, should be protected from light and used within 1 week. Discard any vial if the solution is not colorless. Rx only
Indications & Usage
1 INDICATIONS AND USAGE Levalbuterol Inhalation Solution, USP is indicated for the treatment or prevention of bronchospasm in adults, adolescents, and children 6 years of age and older with reversible obstructive airway disease. Levalbuterol Inhalation Solution, USP is a beta 2 -adrenergic agonist indicated for: Treatment or prevention of bronchospasm in adults, adolescents, and children 6 years of age and older with reversible obstructive airway disease. ( 1 )
Dosage and Administration
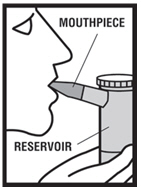
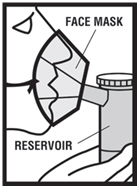
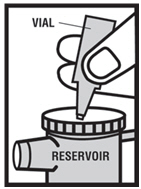
2 DOSAGE AND ADMINISTRATION Levalbuterol Inhalation Solution, USP is for oral inhalation only. Administer by nebulization using a standard jet nebulizer (with a face mask or mouthpiece) connected to an air compressor. Do not exceed recommended dose. FOR ORAL INHALATION ONLY ( 2 ) Children 6-11 years old: 0.31 mg administered three times a day, by nebulization. Routine dosing should not exceed 0.63 mg three times a day. ( 2 ) Adults and Adolescents ≥ 12 years old: 0.63 mg administered three times a day, every 6 to 8 hours, by nebulization. The maximum recommended dose is 1.25 mg three times a day. ( 2 ) For use with a standard jet nebulizer (with a face mask or mouthpiece) connected to an air compressor. ( 2 ) Children 6-11 years old: The recommended dosage of Levalbuterol Inhalation Solution, USP for patients 6-11 years old is 0.31 mg administered three times a day, by nebulization. Routine dosing should not exceed 0.63 mg three times a day. Adults and Adolescents ≥ 12 years old: The recommended starting dosage of Levalbuterol Inhalation Solution, USP for patients 12 years of age and older is 0.63 mg administered three times a day, every 6 to 8 hours, by nebulization. Patients 12 years of age and older with more severe asthma or patients who do not respond adequately to a dose of 0.63 mg of Levalbuterol Inhalation Solution, USP may benefit from a dosage of 1.25 mg three times a day. Patients receiving the highest dose of Levalbuterol Inhalation Solution, USP should be monitored closely for adverse systemic effects, and the risks of such effects should be balanced against the potential for improved efficacy. The use of Levalbuterol Inhalation Solution, USP can be continued as medically indicated to help control recurring bouts of bronchospasm. During this time, most patients gain optimal benefit from regular use of the inhalation solution. If a previously effective dosage regimen fails to provide the usual response this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids. The drug compatibility (physical and chemical), efficacy, and safety of Levalbuterol Inhalation Solution, USP when mixed with other drugs in a nebulizer have not been established. The safety and efficacy of Levalbuterol Inhalation Solution, USP have been established in clinical trials when administered using the PARI LC Jet™ and PARI LC Plus™ nebulizers, and the PARI Master ® Dura-Neb ® 2000 and Dura-Neb ® 3000 compressors. The safety and efficacy of Levalbuterol Inhalation Solution, USP when administered using other nebulizer systems have not been established.
