Drug Catalog - Product Detail
LANSOPRAZOLE DR CP 30MG 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 51991-0772-05 | BRECKENRIDGE | 500 | 30MG | CAPSULE |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
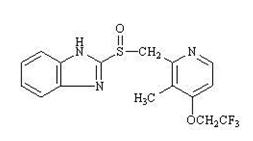
11 DESCRIPTION Lansoprazole delayed-release capsules 15 mg and 30 mg contain lansoprazole as the active ingredient, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C 16 H 14 F 3 N 3 O 2 S with a molecular weight of 369.37. Lansoprazole has the following structure: Lansoprazole is a white to brownish-white odorless crystalline powder which melts with decomposition at approximately 166°C. Lansoprazole is freely soluble in dimethylformamide; soluble in methanol; sparingly soluble in ethanol; slightly soluble in ethyl acetate, dichloromethane and acetonitrile; very slightly soluble in ether; and practically insoluble in hexane and water. Lansoprazole is stable when exposed to light for up to two months. The rate of degradation of the compound in aqueous solution increases with decreasing pH. The degradation half-life of the drug substance in aqueous solution at 25°C is approximately 0.5 hour at pH 5.0 and approximately 18 hours at pH 7.0. Lansoprazole is supplied in delayed-release capsule form, for oral administration in two strengths 15 mg and 30 mg. Lansoprazole delayed-release capsules are available in two strengths: 15 mg and 30 mg of lansoprazole per capsule. Each delayed-release capsule contains lansoprazole delayed-release pellets 8.5% consisting of 15 mg or 30 mg of lansoprazole (active ingredient) and the following inactive ingredients: acetone, hypromellose, isopropyl alcohol, light magnesium carbonate, methacrylic acid copolymer, polyethylene glycol, polysorbate 80, sugar spheres, talc and titanium dioxide. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Lansoprazole delayed-release capsules 15 mg, contains white to off white coloured spherical shaped pellets filled in size '3' capsules with green colour body and pink colour cap, printed NATCO on cap and 15 on body with white ink. Lansoprazole delayed-release capsules 30 mg, contains white to off white coloured spherical shaped pellets filled in size '1' capsules with dark blue colour body and pink colour cap, printed NATCO on cap and 30 on body with white ink. They are available as follows: NDC-51991-771-33 Bottle of 30-15 mg capsules NDC-51991-771-90 Bottle of 90-15 mg capsules NDC-51991-771-05 Bottle of 500-15 mg capsules NDC-51991-771-10 Bottle of 1000-15 mg capsules NDC-51991-772-33 Bottle of 30-30 mg capsules NDC-51991-772-90 Bottle of 90-30 mg capsules NDC-51991-772-01 Bottle of 100-30 mg capsules NDC-51991-772-05 Bottle of 500-30 mg capsules NDC-51991-772-10 Bottle of 1000-30 mg capsules Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). [See USP Controlled Room Temperature].
Indications & Usage
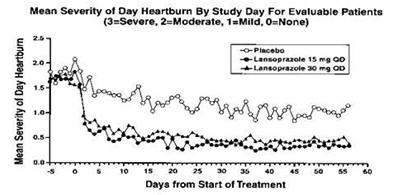
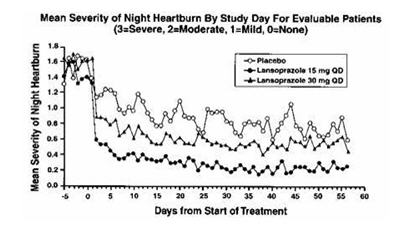
1 INDICATIONS AND USAGE Lansoprazole delayed-release capsule is a proton pump inhibitor (PPI) indicated for: Short-Term Treatment of Active Duodenal Ulcer ( 1.1 ) H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence ( 1.2 ) Maintenance of Healed Duodenal Ulcers ( 1.3 ) Short-Term Treatment of Active Benign Gastric Ulcer ( 1.4 ) Healing of nonsteroidal anti-inflammatory drugs (NSAID)- Associated Gastric Ulcer ( 1.5 ) Risk Reduction of NSAID-Associated Gastric Ulcer ( 1.6 ) Gastroesophageal Reflux Disease (GERD) ( 1.7 ) Maintenance of Healing of Erosive Esophagitis (EE) ( 1.8 ) Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome (ZES) ( 1.9 ) 1.1 Short-Term Treatment of Active Duodenal Ulcer Lansoprazole delayed-release capsules are indicated for short-term treatment (for 4 weeks) for healing and symptom relief of active duodenal ulcer [see Clinical Studies (14) ]. 1.2 H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence Triple Therapy: Lansoprazole delayed-release capsules/amoxicillin/clarithromycin Lansoprazole delayed-release capsules in combination with amoxicillin plus clarithromycin as triple therapy is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one-year history of a duodenal ulcer) to eradicate H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence [see Clinical Studies (14) ]. Please refer to the full prescribing information for amoxicillin and clarithromycin. Dual Therapy: Lansoprazole delayed-release capsules/amoxicillin Lansoprazole delayed-release capsules in combination with amoxicillin as dual therapy is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one-year history of a duodenal ulcer) who are either allergic or intolerant to clarithromycin or in whom resistance to clarithromycin is known or suspected (see the clarithromycin package insert, MICROBIOLOGY section). Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence [see Clinical Studies (14) ]. Please refer to the full prescribing information for amoxicillin. 1.3 Maintenance of Healed Duodenal Ulcers Lansoprazole delayed-release capsules are indicated to maintain healing of duodenal ulcers. Controlled studies do not extend beyond 12 months [see Clinical Studies (14) ]. 1.4 Short-Term Treatment of Active Benign Gastric Ulcer Lansoprazole delayed-release capsules are indicated for short-term treatment (up to 8 weeks) for healing and symptom relief of active benign gastric ulcer [see Clinical Studies (14) ]. 1.5 Healing of NSAID-Associated Gastric Ulcer Lansoprazole delayed-release capsules are indicated for the treatment of NSAID-associated gastric ulcer in patients who continue NSAID use. Controlled studies did not extend beyond 8 weeks [see Clinical Studies (14) ]. 1.6 Risk Reduction of NSAID-Associated Gastric Ulcer Lansoprazole delayed-release capsules are indicated for reducing the risk of NSAID-associated gastric ulcers in patients with a history of a documented gastric ulcer who require the use of an NSAID. Controlled studies did not extend beyond 12 weeks [see Clinical Studies (14) ]. 1.7 Gastroesophageal Reflux Disease (GERD) Short-Term Treatment of Symptomatic GERD Lansoprazole delayed-release capsules are indicated for the treatment of heartburn and other symptoms associated with GERD for up to 8 weeks [see Clinical Studies (14) ]. Short-Term Treatment of Erosive Esophagitis Lansoprazole delayed-release capsules are indicated for short-term treatment (up to 8 weeks) for healing and symptom relief of all grades of erosive esophagitis. For patients who do not heal with lansoprazole delayed-release capsules for 8 weeks (5 to10%), it may be helpful to give an additional 8 weeks of treatment. If there is a recurrence of erosive esophagitis an additional 8-week course of lansoprazole delayed-release capsules may be considered [see Clinical Studies (14) ] . 1.8 Maintenance of Healing of Erosive Esophagitis (EE) Lansoprazole delayed-release capsules are indicated to maintain healing of erosive esophagitis. Controlled studies did not extend beyond 12 months [see Clinical Studies (14) ]. 1.9 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome (ZES) Lansoprazole delayed-release capsules are indicated for the long-term treatment of pathological hypersecretory conditions, including Zollinger-Ellison syndrome [see Clinical Studies (14) ].
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Lansoprazole is available as a capsule, in 15 mg and 30 mg strengths. Directions for use specific to the route and available methods of administration for each of these dosage forms is presented below. Lansoprazole delayed-release capsules should be taken before eating. Lansoprazole products SHOULD NOT BE CRUSHED OR CHEWED. In the clinical trials, antacids were used concomitantly with lansoprazole delayed-release capsules. Indication Dose Frequency Duodenal Ulcers ( 1.1 , 1.3 ) Short-Term Treatment 15 mg Once daily for 4 wks Maintenance of Healed 15 mg Once daily H. pylori Eradication to Reduce Recurrence of Duodenal Ulcer ( 1.2 ) Triple Therapy: Lansoprazole delayed-release capsules 30 mg Twice daily for 10 or 14 days Amoxicillin 1 gram Clarithromycin 500 mg Dual Therapy: Lansoprazole delayed-release capsules 30 mg Three times daily for 14 days Amoxicillin 1 gram Benign Gastric Ulcer ( 1.4 ) Short-Term Treatment 30 mg Once daily up to 8 wks NSAID-associated Gastric Ulcer ( 1.6 ) Healing 30 mg Once daily for 8 wks Risk Reduction 15 mg Once daily up to 12 wks GERD ( 1.7 ) Short-Term Treatment of Symptomatic GERD Short-Term Treatment of EE 15 mg Once daily up to 8 wks 30 mg Once daily up to 8 wks Pediatric ( 8.4 ) (1 to 11 years of age) Short-Term Treatment of Symptomatic GERD and Short-Term Treatment of EE ≤ 30 kg 15mg Once daily up to 12 wks > 30 kg 30 mg Once daily up to 12 wks (12 to 17 years of age) Short-Term Treatment of Symptomatic GERD Nonerosive GERD 15 mg Once daily up to 8 wks EE 30 mg Once daily up to 8 wks Maintenance of Healing of EE ( 1.8 ) Studied for 12 months 15 mg Once daily Pathological Hypersecretory Conditions (i.e., ZES) ( 1.9 ) 60 mg Once daily 2.1 Recommended Dose Indication Recommended Dose Frequency Duodenal Ulcers Short-Term Treatment 15 mg Once daily for 4 weeks Maintenance of Healed 15 mg Once daily H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence Please refer to amoxicillin and clarithromycin full prescribing information for CONTRAINDICATIONS and WARNINGS, and for information regarding dosing in elderly and renally-impaired patients. Triple Therapy: Lansoprazole delayed-release capsules 30 mg Twice daily (q12h) for 10 or 14 days Amoxicillin 1 gram Twice daily (q12h) for 10 or 14 days Clarithromycin 500 mg Twice daily (q12h) for 10 or 14 days Dual Therapy: Lansoprazole delayed-release capsules 30 mg Three times daily (q8h) for 14 days Amoxicillin 1 gram Three times daily (q8h) for 14 days Benign Gastric Ulcer Short-Term Treatment 30 mg Once daily for up to 8 weeks NSAID-associated Gastric Ulcer Healing 30 mg Once daily for 8 weeks Controlled studies did not extend beyond indicated duration. Risk Reduction 15 mg Once daily for up to 12 weeks Gastroesophageal Reflux Disease (GERD) Short-Term Treatment of Symptomatic GERD 15 mg Once daily for up to 8 weeks Short-Term Treatment of Erosive Esophagitis 30 mg Once daily for up to 8 weeks For patients who do not heal with lansoprazole delayed-release capsules for 8 weeks (5–10%), it may be helpful to give an additional 8 weeks of treatment. If there is a recurrence of erosive esophagitis, an additional 8 week course of lansoprazole delayed-release capsules may be considered. Pediatric (1 to 11 years of age) Short-Term Treatment of Symptomatic GERD and Short-Term Treatment of Erosive Esophagitis ≤ 30 kg 15 mg Once daily for up to 12 weeks The lansoprazole delayed-release capsules dose was increased (up to 30 mg twice daily) in some pediatric patients after 2 or more weeks of treatment if they remained symptomatic. For pediatric patients unable to swallow an intact capsule please see Administration Options. > 30 kg 30 mg Once daily for up to 12 weeks (12 to 17 years of age) Short-Term Treatment of Symptomatic GERD Nonerosive GERD 15 mg Once daily for up to 8 weeks Erosive Esophagitis 30 mg Once daily for up to 8 weeks Maintenance of Healing of Erosive Esophagitis 15 mg Once daily Controlled studies did not extend beyond 12 months Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome 60 mg Once daily Varies with individual patient. Recommended adult starting dose is 60 mg once daily. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Dosages up to 90 mg twice daily have been administered. Daily dose of greater than 120 mg should be administered in divided doses. Some patients with Zollinger-Ellison Syndrome have been treated continuously with lansoprazole delayed-release capsules for more than 4 years. Patients should be instructed that if a dose is missed, it should be taken as soon as possible. However, if the next scheduled dose is due, the patient should not take the missed dose, and should be instructed to take the next dose on time. Patients should be instructed not to take 2 doses at one time to make up for a missed dose. 2.2 Special Populations Renal impairment patients and geriatric patients do not require dosage adjustment. However, consider dose adjustment in patients with severe liver impairment [see Use in Specific Populations (8.5 , 8.6 and 8.7) ]. 2.3 Important Administration Information Administration Options Lansoprazole delayed-release capsules - Oral Administration Lansoprazole delayed-release capsules should be swallowed whole. Alternatively, for patients who have difficulty swallowing capsules, lansoprazole delayed-release capsules can be opened and administered as follows: Open capsule. Sprinkle intact granules on one tablespoon of either applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears. Swallow immediately. Lansoprazole delayed-release capsules may also be emptied into a small volume of either apple juice, orange juice or tomato juice and administered as follows: Open capsule. Sprinkle intact granules into a small volume of either apple juice, orange juice or tomato juice (60 mL – approximately 2 ounces). Mix briefly. Swallow immediately. To ensure complete delivery of the dose, the glass should be rinsed with two or more volumes of juice and the contents swallowed immediately. Lansoprazole delayed-release capsules – Nasogastric Tube (≥16 French) Administration For patients who have a nasogastric tube in place, lansoprazole delayed-release capsules can be administered as follows: Open capsule. Mix intact granules into 40 mL of apple juice. DO NOT USE OTHER LIQUIDS. Inject through the nasogastric tube into the stomach. Flush with additional apple juice to clear the tube. USE IN OTHER FOODS AND LIQUIDS HAS NOT BEEN STUDIED CLINICALLY AND IS THEREFORE NOT RECOMMENDED.
