Drug Catalog - Product Detail
LAMOTRIGINE ODT TB 50MG 2X15 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1527-08 | IMPAX GENERICS | 30 | 50MG | NA |
PACKAGE FILES
Generic Name
LAMOTRIGINE
Substance Name
LAMOTRIGINE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200828
Description
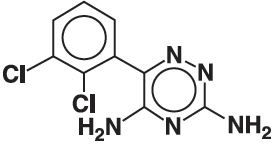
11 DESCRIPTION Lamotrigine, an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)- as -triazine, its molecular formula is C 9 H 7 N 5 Cl 2 , and its molecular weight is 256.09. Lamotrigine is a white to pale cream-colored powder and has a pK a of 5.7. Lamotrigine is very slightly soluble in water (0.17 mg/mL at 25°C) and slightly soluble in 0.1 M HCl (4.1 mg/mL at 25°C). The structural formula is: Lamotrigine orally disintegrating tablets are supplied for oral administration. The tablets contain 25 mg (white to off-white), 50 mg (white to off-white), 100 mg (white to off-white), or 200 mg (white to off-white) of lamotrigine and the following inactive ingredients: amino methacrylate copolymer, aspartame, black currant flavor, colloidal silicon dioxide, crospovidone, magnesium stearate, mannitol, microcrystalline cellulose, peppermint flavor, pregelatinized maize starch, sodium chloride, and stearic acid. Lamotrigine orally disintegrating tablets are formulated using granulation technology in which the lamotrigine drug substance is granulated with stearic acid and eudragit EPO for taste masking. The formulation contains flavor and has a good mouth feel, no grittiness, no after taste, better palatability and gives a desired dissolution profile. Chemical Structure
How Supplied
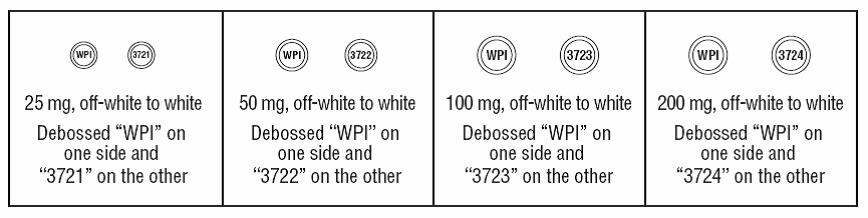
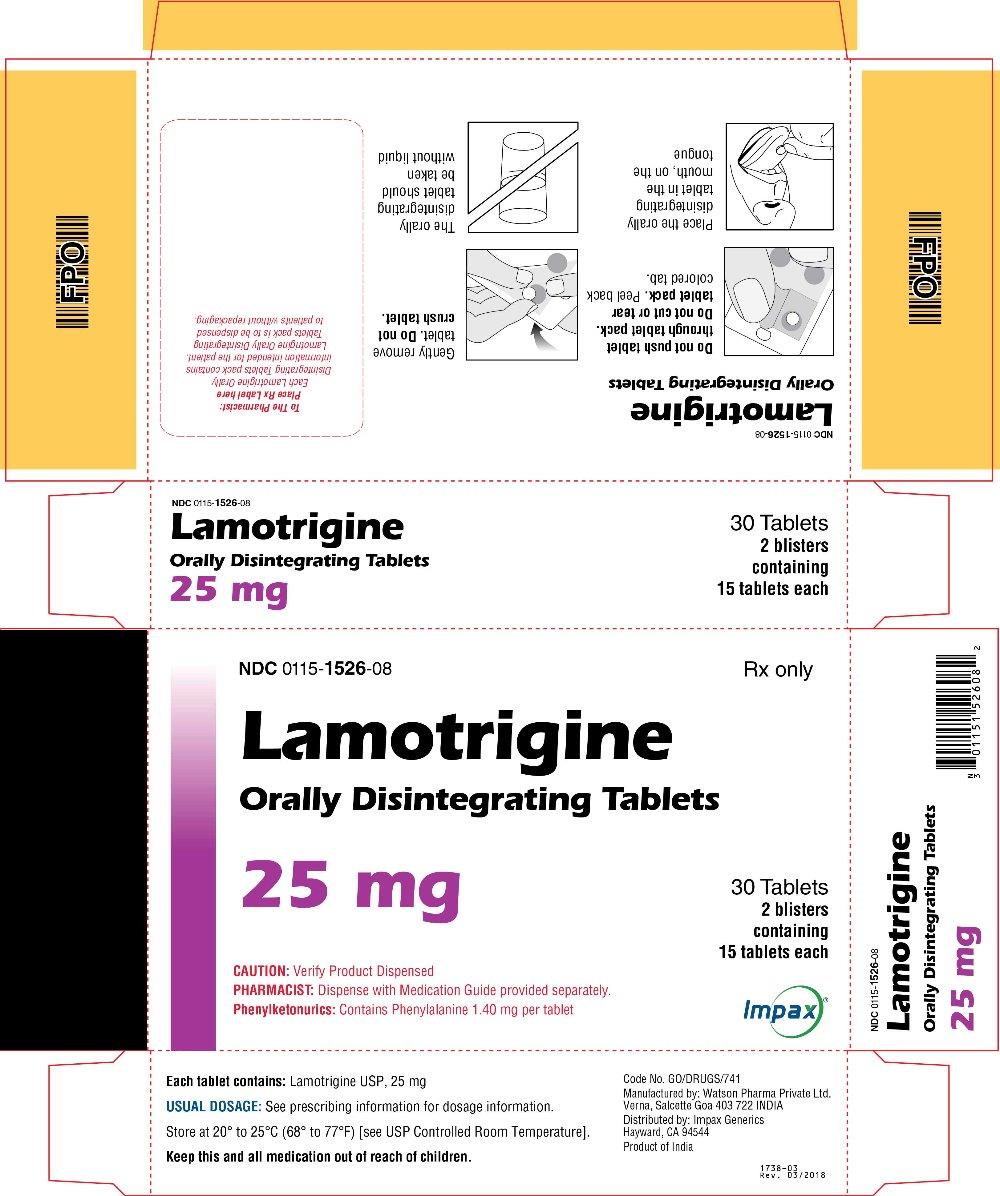
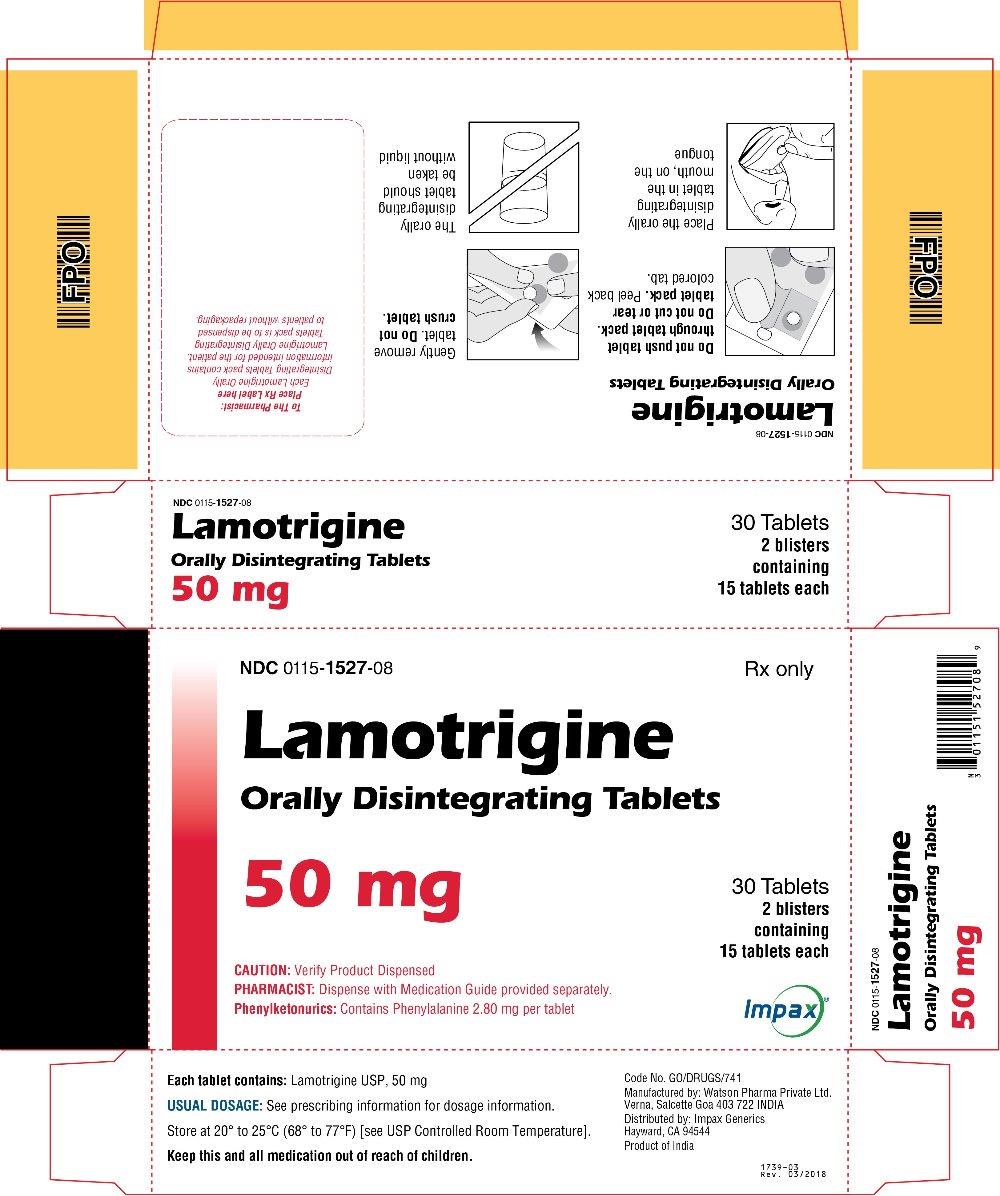
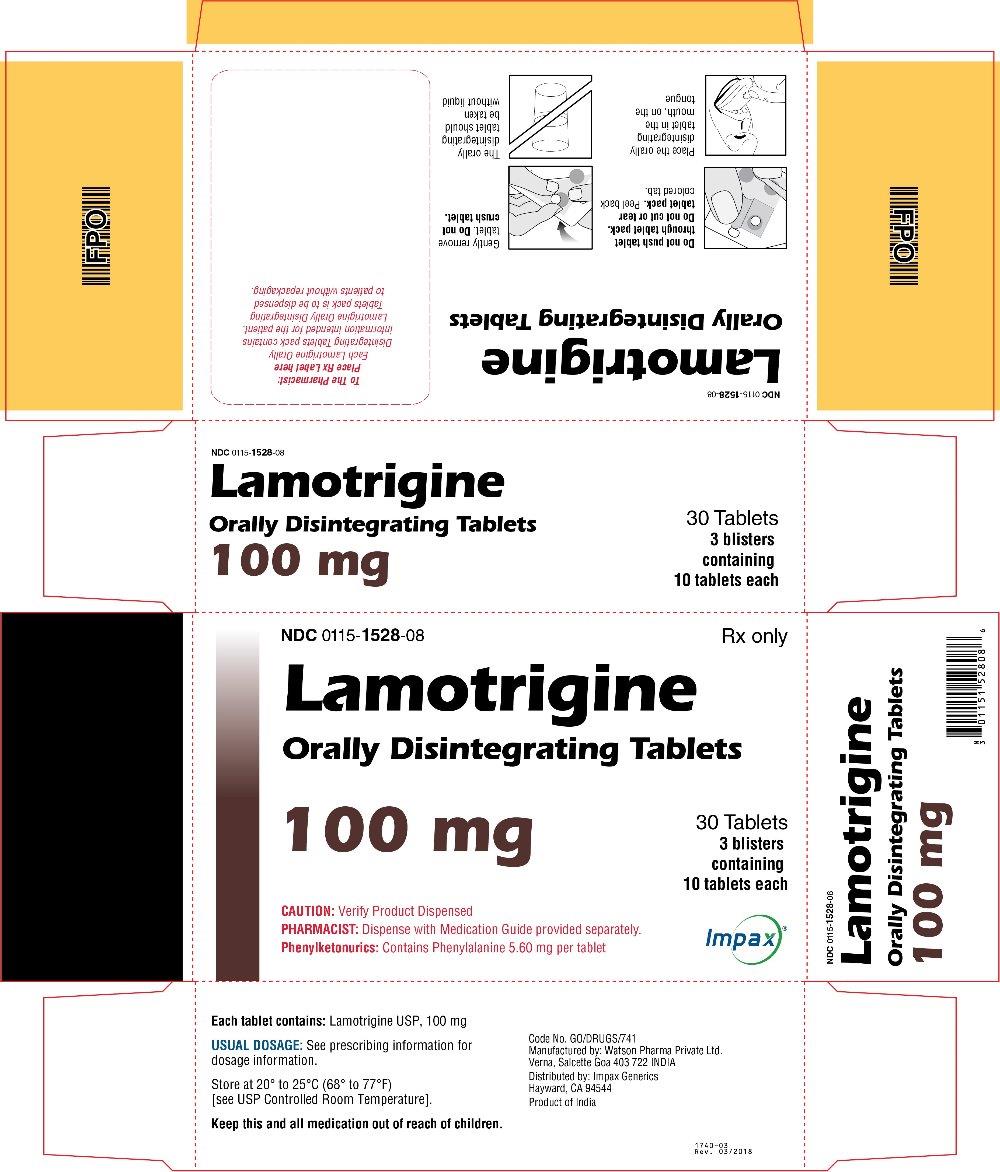
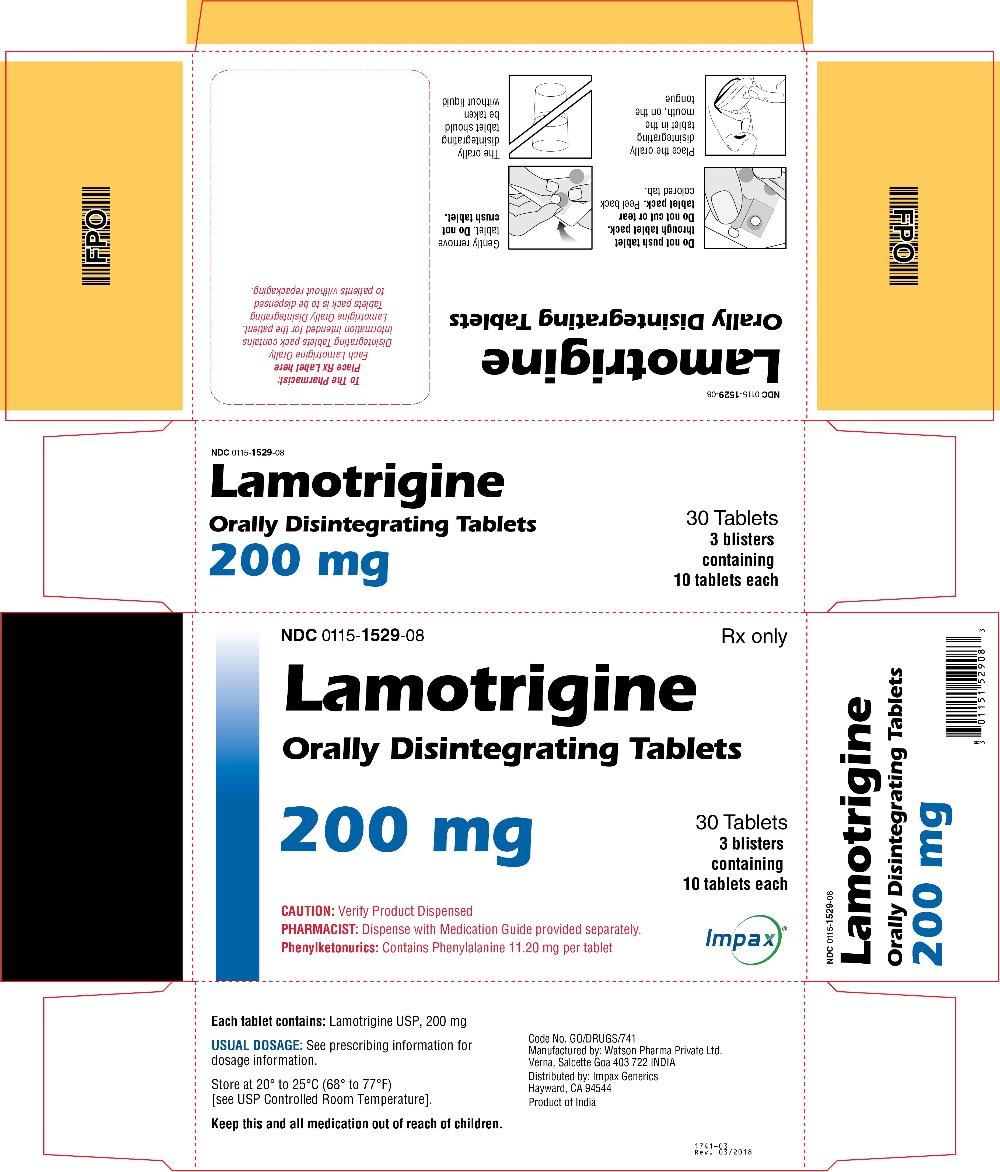
16 HOW SUPPLIED/STORAGE AND HANDLING Lamotrigine Orally Disintegrating Tablets 25 mg, off-white to white circular tablets debossed with “WPI” on one side and “3721” on the other supplied in blisters of 15 (NDC 0115-1526-68) and in cartons of 30 (NDC 0115-1526-08). 50 mg, off-white to white circular tablets debossed with “WPI” on one side and “3722” on the other supplied in blisters of 15 (NDC 0115-1527-68) and in cartons of 30 (NDC 0115-1527-08). 100 mg, off-white to white circular tablets debossed with “WPI” on one side and “3723” on the other supplied in blisters of 10 (NDC 0115-1528-15) and in cartons of 30 (NDC 0115-1528-08). 200 mg, off-white to white circular tablets debossed with “WPI” on one side and “3724” on the other supplied in blisters of 10 (NDC 0115-1529-15) and in cartons of 30 (NDC 0115-1529-08). Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
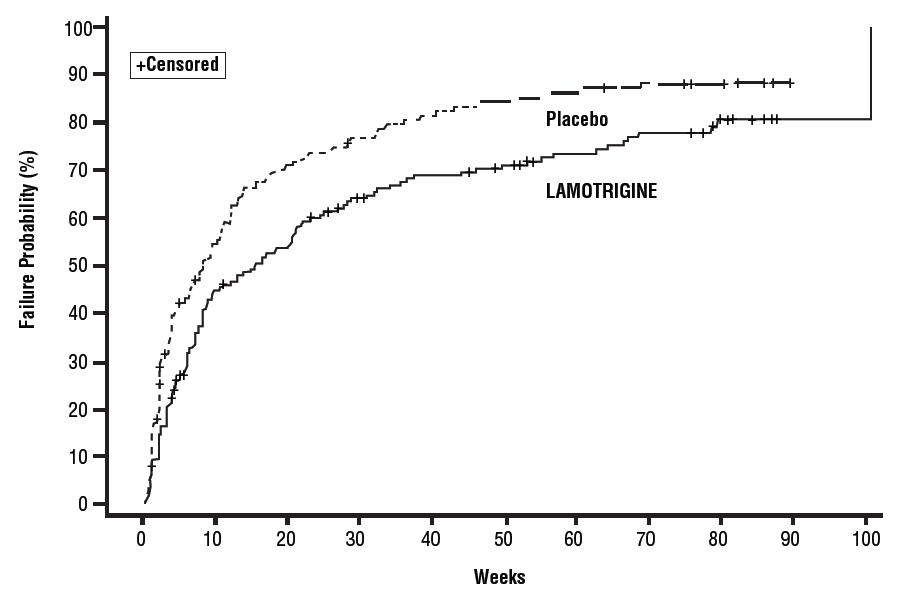
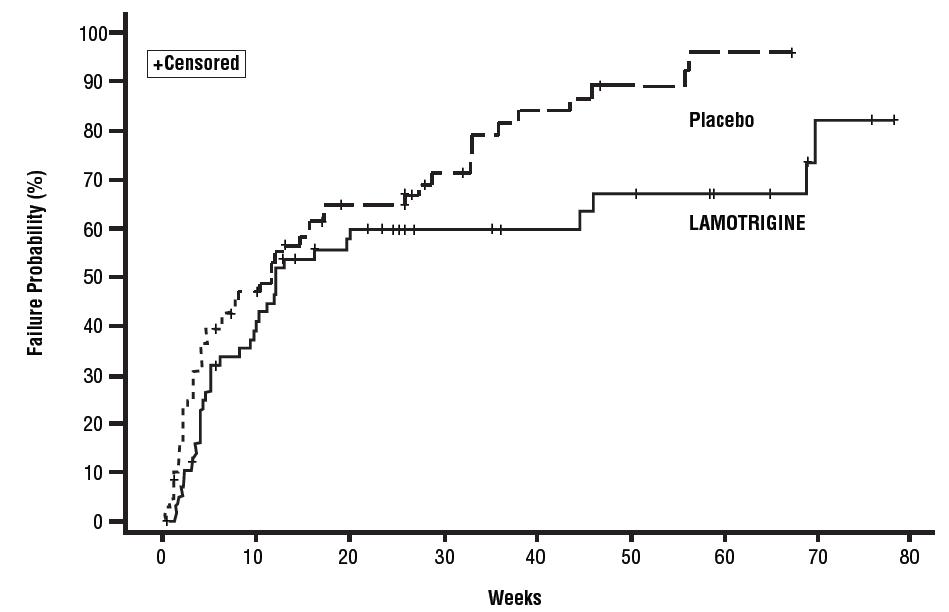
1 INDICATIONS AND USAGE Lamotrigine is indicated for: Epilepsy—adjunctive therapy in patients aged 2 years and older: partial-onset seizures. primary generalized tonic-clonic seizures. generalized seizures of Lennox-Gastaut syndrome ( 1.1 ). Epilepsy—monotherapy in patients aged 16 years and older: Conversion to monotherapy in patients with partial-onset seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single AED. ( 1.1 ) Bipolar Disorder : Maintenance treatment of bipolar I disorder to delay the time to occurrence of mood episodes in patients treated for acute mood episodes with standard therapy. ( 1.2 ) Limitations of Use: Treatment of acute manic or mixed episodes is not recommended. Effectiveness of lamotrigine orally disintegrating tablets in the acute treatment of mood episodes has not been established. 1.1 Epilepsy Adjunctive Therapy Lamotrigine orally disintegrating tablets are indicated as adjunctive therapy for the following seizure types in patients aged 2 years and older: partial-onset seizures primary generalized tonic-clonic (PGTC) seizures generalized seizures of Lennox-Gastaut syndrome Monotherapy Lamotrigine orally disintegrating tablets are indicated for conversion to monotherapy in adults (aged 16 years and older) with partial-onset seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug (AED). Safety and effectiveness of lamotrigine have not been established (1) as initial monotherapy; (2) for conversion to monotherapy from AEDs other than carbamazepine, phenytoin, phenobarbital, primidone, or valproate; or (3) for simultaneous conversion to monotherapy from 2 or more concomitant AEDs. 1.2 Bipolar Disorder Lamotrigine orally disintegrating tablets are indicated for the maintenance treatment of bipolar I disorder to delay the time to occurrence of mood episodes (depression, mania, hypomania, mixed episodes) in patients treated for acute mood episodes with standard therapy [see Clinical Studies (14.1) ] . Limitations of Use Treatment of acute manic or mixed episodes is not recommended. Effectiveness of lamotrigine in the acute treatment of mood episodes has not been established.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Dosing is based on concomitant medications, indication, and patient age. ( 2.1 , 2.2 , 2.3 , 2.4 ) To avoid an increased risk of rash, the recommended initial dose and subsequent dose escalations should not be exceeded. ( 2.1 , 16 ) Do not restart lamotrigine orally disintegrating tablets in patients who discontinued due to rash unless the potential benefits clearly outweigh the risks. ( 2.1 , 5.1 ) Adjustments to maintenance doses will be necessary in most patients starting or stopping estrogen-containing oral contraceptives. ( 2.1 , 5.7 ) Discontinuation: Taper over a period of at least 2 weeks (approximately 50% dose reduction per week). ( 2.1 , 5.8 ) Epilepsy: Adjunctive therapy—See Table 1 for patients older than 12 years and Tables 2 and 3 for patients aged 2 to 12 years. ( 2.2 ) Conversion to monotherapy—See Table 4. ( 2.3 ) Bipolar Disorder: See Tables 5 and 6. ( 2.4 ) 2.1 General Dosing Considerations Rash There are suggestions, yet to be proven, that the risk of severe, potentially life-threatening rash may be increased by (1) coadministration of lamotrigine orally disintegrating tablets with valproate, (2) exceeding the recommended initial dose of lamotrigine orally disintegrating tablets, or (3) exceeding the recommended dose escalation for lamotrigine orally disintegrating tablets. However, cases have occurred in the absence of these factors [see Boxed Warning ] . Therefore, it is important that the dosing recommendations be followed closely. The risk of nonserious rash may be increased when the recommended initial dose and/or the rate of dose escalation of lamotrigine orally disintegrating tablets is exceeded and in patients with a history of allergy or rash to other AEDs. It is recommended that lamotrigine orally disintegrating tablets not be restarted in patients who discontinued due to rash associated with prior treatment with lamotrigine, unless the potential benefits clearly outweigh the risks. If the decision is made to restart a patient who has discontinued lamotrigine, the need to restart with the initial dosing recommendations should be assessed. The greater the interval of time since the previous dose, the greater consideration should be given to restarting with the initial dosing recommendations. If a patient has discontinued lamotrigine for a period of more than 5 half-lives, it is recommended that initial dosing recommendations and guidelines be followed. The half-life of lamotrigine is affected by other concomitant medications [see Clinical Pharmacology (12.3) ] . Lamotrigine Added to Drugs Known to Induce or Inhibit Glucuronidation Because lamotrigine is metabolized predominantly by glucuronic acid conjugation, drugs that are known to induce or inhibit glucuronidation may affect the apparent clearance of lamotrigine. Drugs that induce glucuronidation include carbamazepine, phenytoin, phenobarbital, primidone, rifampin, estrogen-containing oral contraceptives, and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir. Valproate inhibits glucuronidation. For dosing considerations for lamotrigine orally disintegrating tablets in patients on estrogen-containing contraceptives and atazanavir/ritonavir, see below and Table 13. For dosing considerations for lamotrigine orally disintegrating tablets in patients on other drugs known to induce or inhibit glucuronidation, see Table 1, Table 2, Table 5, Table 6, and Table 13. Target Plasma Levels for Patients with Epilepsy or Bipolar Disorder A therapeutic plasma concentration range has not been established for lamotrigine. Dosing of lamotrigine orally disintegrating tablets should be based on therapeutic response [see Clinical Pharmacology (12.3) ] . Women Taking Estrogen-Containing Oral Contraceptives Starting Lamotrigine Orally Disintegrating Tablets in Women Taking Estrogen-Containing Oral Contraceptives: Although estrogen-containing oral contraceptives have been shown to increase the clearance of lamotrigine [see Clinical Pharmacology (12.3)] , no adjustments to the recommended dose-escalation guidelines for lamotrigine orally disintegrating tablets should be necessary solely based on the use of estrogen-containing oral contraceptives. Therefore, dose escalation should follow the recommended guidelines for initiating adjunctive therapy with lamotrigine orally disintegrating tablets based on the concomitant AED or other concomitant medications (see Table 1, Table 5 and Table 7). See below for adjustments to maintenance doses of lamotrigine in women taking estrogen-containing oral contraceptives. Adjustments to the Maintenance Dose of Lamotrigine Orally Disintegrating Tablets In Women Taking Estrogen-Containing Oral Contraceptives: (1) Taking Estrogen-Containing Oral Contraceptives: In women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation [see Drug Interactions (7) , Clinical Pharmacology (12.3) ] , the maintenance dose of lamotrigine orally disintegrating tablets will in most cases need to be increased by as much as 2-fold over the recommended target maintenance dose to maintain a consistent lamotrigine plasma level. (2) Starting Estrogen-Containing Oral Contraceptives: In women taking a stable dose of lamotrigine orally disintegrating tablets and not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation [see Drug Interactions (7) , Clinical Pharmacology (12.3) ] , the maintenance dose will in most cases need to be increased by as much as 2-fold to maintain a consistent lamotrigine plasma level. The dose increases should begin at the same time that the oral contraceptive is introduced and continue, based on clinical response, no more rapidly than 50 to 100 mg/day every week. Dose increases should not exceed the recommended rate (see Table 1 and Table 5) unless lamotrigine plasma levels or clinical response support larger increases. Gradual transient increases in lamotrigine plasma levels may occur during the week of inactive hormonal preparation (pill-free week), and these increases will be greater if dose increases are made in the days before or during the week of inactive hormonal preparation. Increased lamotrigine plasma levels could result in additional adverse reactions, such as dizziness, ataxia, and diplopia. If adverse reactions attributable to lamotrigine consistently occur during the pill-free week, dose adjustments to the overall maintenance dose may be necessary. Dose adjustments limited to the pill-free week are not recommended. For women taking lamotrigine in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation [see Drug Interactions (7) , Clinical Pharmacology (12.3) ] , no adjustment to the dose of lamotrigine orally disintegrating tablets should be necessary. (3) Stopping Estrogen-Containing Oral Contraceptives: In women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation [see Drug Interactions (7), Clinical Pharmacology (12.3)] , the maintenance dose of lamotrigine orally disintegrating tablets will in most cases need to be decreased by as much as 50% in order to maintain a consistent lamotrigine plasma level. The decrease in dose of lamotrigine orally disintegrating tablets should not exceed 25% of the total daily dose per week over a 2-week period, unless clinical response or lamotrigine plasma levels indicate otherwise [see Clinical Pharmacology (12.3) ] . In women taking lamotrigine in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation [see Drug Interactions (7) , Clinical Pharmacology (12.3) ] , no adjustment to the dose of lamotrigine orally disintegrating tablets should be necessary. Women and Other Hormonal Contraceptive Preparations or Hormone Replacement Therapy The effect of other hormonal contraceptive preparations or hormone replacement therapy on the pharmacokinetics of lamotrigine has not been systematically evaluated. It has been reported that ethinylestradiol, not progestogens, increased the clearance of lamotrigine up to 2-fold, and the progestin-only pills had no effect on lamotrigine plasma levels. Therefore, adjustments to the dosage of lamotrigine orally disintegrating tablets in the presence of progestogens alone will likely not be needed. Patients Taking Atazanavir/Ritonavir While atazanavir/ritonavir does reduce the lamotrigine plasma concentration, no adjustments to the recommended dose-escalation guidelines for lamotrigine orally disintegrating tablets should be necessary solely based on the use of atazanavir/ritonavir. Dose escalation should follow the recommended guidelines for initiating adjunctive therapy with lamotrigine orally disintegrating tablets based on concomitant AED or other concomitant medications (see Table 1, Table 2, and Table 5). In patients already taking maintenance doses of lamotrigine and not taking glucuronidation inducers, the dose of lamotrigine orally disintegrating tablets may need to be increased if atazanavir/ritonavir is added, or decreased if atazanavir/ritonavir is discontinued [see Clinical Pharmacology (12.3) ] . Patients with Hepatic Impairment Experience in patients with hepatic impairment is limited. Based on a clinical pharmacology study in 24 patients with mild, moderate, and severe liver impairment [see Use in Specific Populations (8.6) , Clinical Pharmacology (12.3) ] , the following general recommendations can be made. No dosage adjustment is needed in patients with mild liver impairment. Initial, escalation, and maintenance doses should generally be reduced by approximately 25% in patients with moderate and severe liver impairment without ascites and 50% in patients with severe liver impairment with ascites. Escalation and maintenance doses may be adjusted according to clinical response. Patients with Renal Impairment Initial doses of lamotrigine orally disintegrating tablets should be based on patients' concomitant medications (see Table1, Table 2, Table 3 and Table 5); reduced maintenance doses may be effective for patients with significant renal impairment [see Use in Specific Populations (8.7) , Clinical Pharmacology (12.3) ] . Few patients with severe renal impairment have been evaluated during chronic treatment with lamotrigine. Because there is inadequate experience in this population, lamotrigine orally disintegrating tablets should be used with caution in these patients. Discontinuation Strategy Epilepsy: For patients receiving lamotrigine orally disintegrating tablets in combination with other AEDs, a reevaluation of all AEDs in the regimen should be considered if a change in seizure control or an appearance or worsening of adverse reactions is observed. If a decision is made to discontinue therapy with lamotrigine orally disintegrating tablets, a step-wise reduction of dose over at least 2 weeks (approximately 50% per week) is recommended unless safety concerns require a more rapid withdrawal [see Warnings and Precautions (5.8) ] . Discontinuing carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation should prolong the half-life of lamotrigine; discontinuing valproate should shorten the half-life of lamotrigine. Bipolar Disorder: In the controlled clinical trials, there was no increase in the incidence, type, or severity of adverse reactions following abrupt termination of lamotrigine. In clinical development program in adults with bipolar disorder, 2 patients experienced seizures shortly after abrupt withdrawal of lamotrigine. Discontinuation of lamotrigine orally disintegrating tablets should involve a step-wise reduction of dose over at least 2 weeks (approximately 50% per week) unless safety concerns require a more rapid withdrawal [see Warnings and Precautions (5.8) ] . 2.2 Epilepsy – Adjunctive Therapy This section provides specific dosing recommendations for patients older than 12 years and patients aged 2 to 12 years. Within each of these age-groups, specific dosing recommendations are provided depending upon concomitant AEDs or other concomitant medications (see Table 1 for patients older than 12 years and Table 2 for patients aged 2 to 12 years). A weight-based dosing guide for patients aged 2 to 12 years on concomitant valproate is provided in Table 3. Patients Older Than 12 Years Recommended dosing guidelines are summarized in Table 1. Table 1. Escalation Regimen for Lamotrigine Orally Disintegrating Tablets in Patients Older Than 12 Years with Epilepsy For Patients TAKING Valproate Valproate has been shown to inhibit glucuronidation and decrease the apparent clearance of lamotrigine [see Drug Interactions (7) , Clinical Pharmacology (12.3) ]. For Patients NOT TAKING Carbamazepine, Phenytoin, Phenobarbital, Primidone These drugs induce glucuronidation and increase clearance [see Drug Interactions (7) Clinical Pharmacology (12.3) ]. Other drugs that have similar effects include estrogen-containing oral contraceptives [See Drug Interactions (7) , Clinical Pharmacology (12.3) ]. Dosing recommendations for oral contraceptives can be found in General Dosing Considerations [see Dosage and Administration (2.1) ]. Patients on rifampin, or other drugs that induce lamotrigine glucuronidation and increase clearance, should follow the same dosing titration/maintenance regimen as that used with anticonvulsants that have this effect. or Valproate For Patients TAKING Carbamazepine, Phenytoin, Phenobarbital, or Primidone and NOT TAKING Valproate Weeks 1 and 2 25 mg every other day 25 mg every day 50 mg/day Weeks 3 and 4 25 mg every day 50 mg/day 100 mg/day (in 2 divided doses) Week 5 onwards to maintenance Increase by 25 to 50 mg/day every 1 to 2 weeks Increase by 50 mg/day every 1 to 2 weeks Increase by 100 mg/day every 1 to 2 weeks Usual maintenance dose 100 to 200 mg/day with valproate alone 100 to 400 mg/day with valproate and other drugs that induce glucuronidation (in 1 or 2 divided doses) 225 to 375 mg/day (in 2 divided doses) 300 to 500 mg/day (in 2 divided doses) Patients Aged 2 to 12 Years Recommended dosing guidelines are summarized in Table 2. Lower starting doses and slower dose escalations than those used in clinical trials are recommended because of the suggestion that the risk of rash may be decreased by lower starting doses and slower dose escalations. Therefore, maintenance doses will take longer to reach in clinical practice than in clinical trials. It may take several weeks to months to achieve an individualized maintenance dose. Maintenance doses in patients weighing less than 30 kg, regardless of age or concomitant AED, may need to be increased as much as 50%, based on clinical response. In Patients TAKING Valproate In Patients NOT TAKING Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate In Patients TAKING Carbamazepine, Phenytoin, Phenobarbital, or Primidoneand NOT TAKING Valproate Weeks 1 and 2 0.15 mg/kg/day in 1 or 2 divided doses, rounded down to the nearest whole tablet (see Table 3 for weight-based dosing guide) 0.3 mg/kg/day in 1 or 2 divided doses, rounded down to the nearest whole tablet 0.6 mg/kg/day in 2 divided doses, rounded down to the nearest whole tablet Weeks 3 and 4 0.3 mg/kg/day in 1 or 2 divided doses, rounded down to the nearest whole tablet (see Table 3 for weight-based dosing guide) 0.6 mg/kg/day in 2 divided doses, rounded down to the nearest whole tablet 1.2 mg/kg/day in 2 divided doses, rounded down to the nearest whole tablet Week 5 onward to maintenance The dose should be increased every 1 to2 weeks as follows: calculate 0.3 mg/kg/day, round this amount down to the nearest whole tablet, and add this amount to the previously administered daily dose The dose should be increased every 1 to 2 weeks as follows: calculate 0.6 mg/kg/day, round this amount down to the nearest whole tablet, and add this amount to the previously administered daily dose The dose should be increased every 1 to 2 weeks as follows: calculate 1.2 mg/kg/day, round this amount down to the nearest whole tablet, and add this amount to the previously administered daily dose Usual Maintenance Dose 1 to 5 mg/kg/day (maximum 200 mg/day in 1 or 2 divided doses) 1 to 3 mg/kg/day with valproate alone 4.5 to 7.5 mg/kg/day (maximum 300 mg/day in 2 divided doses) 5 to 15 mg/kg/day (maximum 400 mg/day in 2 divided doses) Maintenance dose in patients less than 30 kg May need to be increased by as much as 50%, based on clinical response May need to be increased by as much as 50%, based on clinical response May need to be increased by as much as 50%, based on clinical response Note: Only whole tablets should be used for dosing. Table 3. The Initial Weight-Based Dosing Guide for Patients Aged 2 to 12 Years Taking Valproate (Weeks 1 to 4) with Epilepsy If the patient’s weight is Give this daily dose, using the most appropriate combination of lamotrigine orally disintegrating 2-mg and 5-mg tablets Greater than And less than Weeks 1 and 2 Weeks 3 and 4 6.7 kg 14 kg 2 mg every other day 2 mg every day 14.1 kg 27 kg 2 mg every day 4 mg every day 27.1 kg 34 kg 4 mg every day 8 mg every day 34.1 kg 40 kg 5 mg every day 10 mg every day Usual Adjunctive Maintenance Dose for Epilepsy The usual maintenance doses identified in Tables 1 and 2 are derived from dosing regimens employed in the placebo-controlled adjunctive studies in which the efficacy of lamotrigine was established. In patients receiving multidrug regimens employing carbamazepine, phenytoin, phenobarbital, or primidone without valproate , maintenance doses of adjunctive lamotrigine as high as 700 mg/day have been used. In patients receiving valproate alo ne, maintenance doses of adjunctive lamotrigine as high as 200 mg/day have been used. The advantage of using doses above those recommended in Table 1 through Table 4 has not been established in controlled trials. 2.3 Epilepsy – Conversion From Adjunctive Therapy to Monotherapy The goal of the transition regimen is to attempt to maintain seizure control while mitigating the risk of serious rash associated with the rapid titration of lamotrigine orally disintegrating tablets. The recommended maintenance dose of lamotrigine orally disintegrating tablets as monotherapy is 500 mg/day given in 2 divided doses. To avoid an increased risk of rash, the recommended initial dose and subsequent dose escalations of lamotrigine orally disintegrating tablets should not be exceeded [see Boxed Warning ] . Conversion From Adjunctive Therapy with Carbamazepine, Phenytoin, Phenobarbital, or Primidone to Monotherapy with Lamotrigine Orally Disintegrating Tablets After achieving a dose of 500 mg/day of lamotrigine orally disintegrating tablets using the guidelines in Table 1, the concomitant enzyme-inducing AED should be withdrawn by 20% decrements each week over a 4-week period. The regimen for the withdrawal of the concomitant AED is based on experience gained in the controlled monotherapy clinical trial. Conversion From Adjunctive Therapy with Valproate to Monotherapy with Lamotrigine Orally Disintegrating Tablets The conversion regimen involves 4 steps outlined in Table 4. Table 4. Conversion From Adjunctive Therapy with Valproate to Monotherapy with Lamotrigine Orally Disintegrating Tablets in Patients Aged 16 Years and Older with Epilepsy Lamotrigine Orally Disintegrating Tablets Valproate Step 1 Achieve a dose of 200 mg/day according to guidelines in Table 1 Maintain established stable dose. Step 2 Maintain at 200 mg/day. Decrease dose by decrements no greater than 500 mg/day/week to 500 mg/day and then maintain for 1 week. Step 3 Increase to 300 mg/day and maintain for 1 week. Simultaneously decrease to 250 mg/day and maintain for 1 week. Step 4 Increase by 100 mg/day every week to achieve maintenance dose of 500 mg/day. Discontinue. Conversion From Adjunctive Therapy with Antiepileptic Drugs Other Than Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate to Monotherapy with Lamotrigine Orally Disintegrating Tablets No specific dosing guidelines can be provided for conversion to monotherapy with lamotrigine orally disintegrating tablets with AEDs other than carbamazepine, phenobarbital, phenytoin, primidone, or valproate. 2.4 Bipolar Disorder The goal of maintenance treatment with lamotrigine orally disintegrating tablets is to delay the time to occurrence of mood episodes (depression, mania, hypomania, mixed episodes) in patients treated for acute mood episodes with standard therapy [see Indications and Usage (1)] . Patients taking lamotrigine orally disintegrating tablets for more than 16 weeks should be periodically reassessed to determine the need for maintenance treatment. Adults The target dose of lamotrigine orally disintegrating tablets is 200 mg/day (100 mg/day in patients taking valproate, which decreases the apparent clearance of lamotrigine, and 400 mg/day in patients not taking valproate and taking either carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitor lopinavir/ritonavir that increase the apparent clearance of lamotrigine). In the clinical trials, doses up to 400 mg/day as monotherapy were evaluated; however, no additional benefit was seen at 400 mg/day compared with 200 mg/day [see Clinical Studies (14.2) ] . Accordingly, doses above 200 mg/day are not recommended. Treatment with lamotrigine orally disintegrating tablets is introduced, based on concurrent medications, according to the regimen outlined in Table 5. If other psychotropic medications are withdrawn following stabilization, the dose of lamotrigine orally disintegrating tablets should be adjusted. In patients discontinuing valproate, the dose of lamotrigine orally disintegrating tablets should be doubled over a 2-week period in equal weekly increments (see Table 6). In patients discontinuing carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation, the dose of lamotrigine orally disintegrating tablets should remain constant for the first week and then should be decreased by half over a 2-week period in equal weekly decrements (see Table 6). The dose of lamotrigine orally disintegrating tablets may then be further adjusted to the target dose (200 mg) as clinically indicated. If other drugs are subsequently introduced, the dose of lamotrigine orally disintegrating tablets may need to be adjusted. In particular, the introduction of valproate requires reduction in the dose of lamotrigine orally disintegrating tablets [see Drug Interactions (7) , Clinical Pharmacology (12.3) ] . To avoid an increased risk of rash, the recommended initial dose and subsequent dose escalations of lamotrigine orally disintegrating tablets should not be exceeded [see Boxed Warning ] . Table 5. Escalation Regimen for Lamotrigine Orally Disintegrating Tablets for Patients with Bipolar Disorder In Patients TAKING Valproate In Patients NOT TAKING Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate In Patients TAKING Carbamazepine, Phenytoin, Phenobarbital, or Primidone, and NOT TAKING Valproate Weeks 1 and 2 25 mg every other day 25 mg daily 50 mg daily Weeks 3 and 4 25 mg daily 50 mg daily 100 mg daily, in divided doses Week 5 50 mg daily 100 mg daily 200 mg daily, in divided doses Week 6 100 mg daily 200 mg daily 300 mg daily, in divided doses Week 7 100 mg daily 200 mg daily up to 400 mg daily, in divided doses Table 6. Dosage Adjustments to Lamotrigine Orally Disintegrating Tablets for Patients With Bipolar Disorder Following Discontinuation of Psychotropic Medications Discontinuation of Psychotropic Drugs (excluding Valproate, Carbamazepine, Phenytoin, Phenobarbital, or Primidone After Discontinuation of Valproate After Discontinuation of Carbamazepine, Phenytoin, Phenobarbital, or Primidone Current dose of lamotrigine orally disintegrating tablets (mg/day) 100 Current dose of lamotrigine orally disintegrating tablets (mg/day) 400 Week 1 Maintain current dose of lamotrigine orally disintegrating tablets 150 400 Week 2 Maintain current dose of lamotrigine orally disintegrating tablets 200 300 Week 3 onward Maintain current dose of lamotrigine orally disintegrating tablets 200 200 2.6 Administration of Lamotrigine Orally Disintegrating Tablets Lamotrigine orally disintegrating tablets should be placed onto the tongue and moved around in the mouth. The tablet will disintegrate rapidly, can be swallowed with or without water, and can be taken with or without food.
