Drug Catalog - Product Detail
LABETALOL HCL IV SOLN 5 MG/ML 40 ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
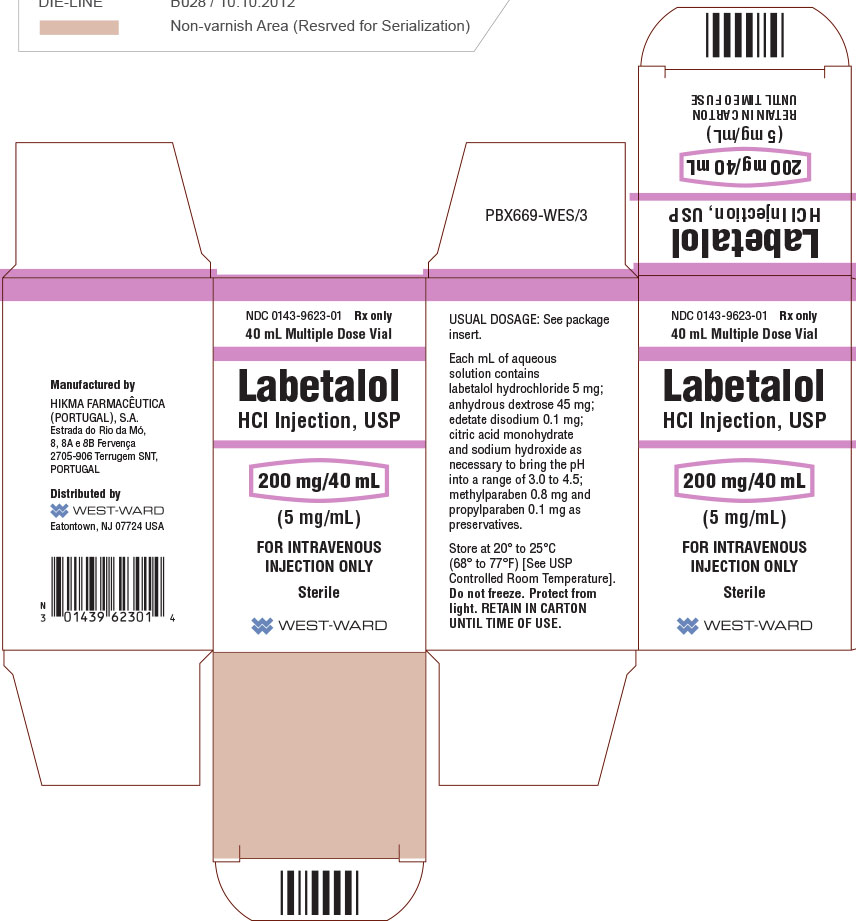
| 00143-9623-01 | HIKMA PHARMACEUTICALS USA | 40 | 5MG/ML | SOLUTION |
PACKAGE FILES


Generic Name
LABETALOL HYDROCHLORIDE
Substance Name
LABETALOL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAVENOUS
Application Number
ANDA075303
Description
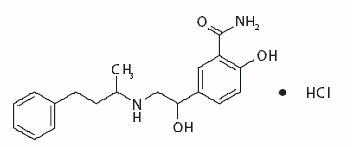
DESCRIPTION Labetalol Hydrochloride Injection, USP is an adrenergic receptor blocking agent that has both selective alpha 1 -adrenergic and nonselective beta-adrenergic receptor blocking actions in a single substance. Labetalol hydrochloride (HCl) is a racemate chemically designated as 5-[1-Hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-salicylamide monohydrochloride and it has the following structural formula: Labetalol HCl has the molecular formula C 19 H 24 N 2 O 3 •HCl and a molecular weight of 364.87. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R' stereoisomer, makes up 25% of racemic labetalol. Labetalol HCl is a white or off-white crystalline powder, soluble in water. Labetalol HCl Injection, USP is a clear, colorless to light yellow, aqueous, sterile, isotonic solution for intravenous injection. It has a pH range of 3 to 4. Each milliliter contains 5 mg of labetalol HCl, 45 mg of anhydrous dextrose, 0.1 mg of edetate disodium; 0.8 mg of methylparaben and 0.1 mg of propylparaben as preservatives; and citric acid monohydrate and sodium hydroxide, as necessary, to bring the solution into the pH range. chemical formula
How Supplied
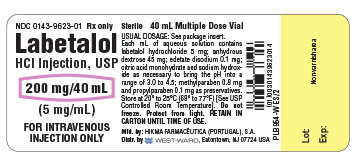
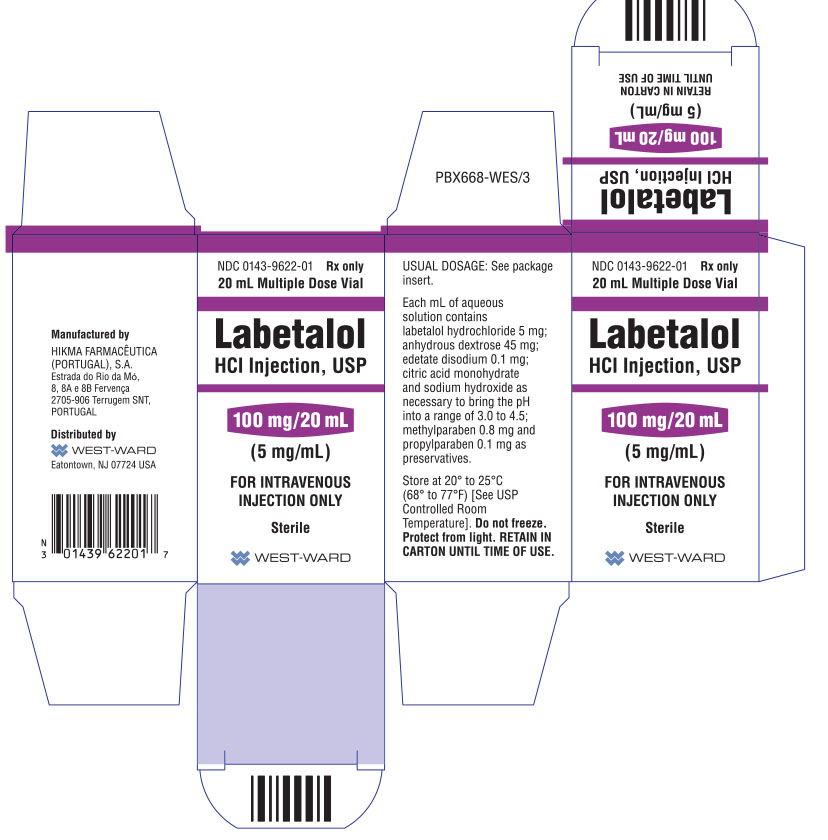
HOW SUPPLIED Labetalol HCl Injection, USP, 5 mg/mL, is supplied in 20 mL (100 mg) multidose vials, individually-boxed (NDC 0143-9622-01) and 40 mL (200 mg) multidose vials, individually-boxed (NDC 0143-9623-01) . Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Do not freeze. Protect from light. To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch . For Product Inquiry call 1-877-845-0689. Manufactured by: HIKMA FARMACÊUTICA (PORTUGAL), S.A. Estrada do Rio da Mó, 8, 8A e 8B – Fervença – 2705-906 Terrugem SNT, PORTUGAL Distributed by: Hikma Pharmaceuticals USA Inc. Berkeley Heights, NJ 07922 Revised January 2020 PIN424-WES/2
Indications & Usage
INDICATIONS AND USAGE Labetalol HCl Injection, USP is indicated for control of blood pressure in severe hypertension.
Dosage and Administration
DOSAGE AND ADMINISTRATION Labetalol HCl injection is intended for intravenous use in hospitalized patients. DOSAGE MUST BE INDIVIDUALIZED depending upon the severity of hypertension and the response of the patient during dosing. Patients should always be kept in a supine position during the period of intravenous drug administration. A substantial fall in blood pressure on standing should be expected in these patients. The patient’s ability to tolerate an upright position should be established before permitting any ambulation, such as using toilet facilities. Either of two methods of administration of labetalol HCl injection may be used: a) repeated intravenous injection, or b) slow continuous infusion. Repeated Intravenous Injection Initially, labetalol HCl injection should be given in a 20 mg dose (which corresponds to 0.25 mg/kg for an 80 kg patient) by slow intravenous injection over a 2 minute period. Immediately before the injection and at 5 and 10 minutes after injection, supine blood pressure should be measured to evaluate response. Additional injections of 40 or 80 mg can be given at 10 minute intervals until a desired supine blood pressure is achieved or a total of 300 mg of labetalol HCl injection has been injected. The maximum effect usually occurs within 5 minutes of each injection. Slow Continuous Infusion Labetalol HCl injection is prepared for continuous intravenous infusion by diluting the vial contents with commonly used intravenous fluids (see below). Examples of two methods of preparing the infusion solution are: The contents of either two 20 mL vials (40 mL), or one 40 mL vial, are added to 160 mL of a commonly used intravenous fluid such that the resultant 200 mL of solution contains 200 mg of labetalol HCl, 1 mg/mL. The diluted solution should be administered at a rate of 2 mL/min to deliver 2 mg/min. Alternatively, the contents of either two 20 mL vials (40 mL), or one 40 mL vial, of labetalol HCl injection are added to 250 mL of a commonly used intravenous fluid. The resultant solution will contain 200 mg of labetalol HCl, approximately 2 mg/3 mL. The diluted solution should be administered at a rate of 3 mL/min to deliver approximately 2 mg/min. The rate of infusion of the diluted solution may be adjusted according to the blood pressure response, at the discretion of the physician. To facilitate a desired rate of infusion, the diluted solution can be infused using a controlled administration mechanism, e.g., graduated burette or mechanically driven infusion pump. Since the half-life of labetalol is 5 to 8 hours, steady-state blood levels (in the face of a constant rate of infusion) would not be reached during the usual infusion time period. The infusion should be continued until a satisfactory response is obtained and should then be stopped and oral labetalol HCl started (see below). The effective intravenous dose is usually in the range of 50 to 200 mg. A total dose of up to 300 mg may be required in some patients. Blood Pressure Monitoring The blood pressure should be monitored during and after completion of the infusion or intravenous injection. Rapid or excessive falls in either systolic or diastolic blood pressure during intravenous treatment should be avoided. In patients with excessive systolic hypertension, the decrease in systolic pressure should be used as an indicator of effectiveness in addition to the response of the diastolic pressure. Initiation of Dosing with Labetalol HCl Tablets Subsequent oral dosing with labetalol HCl tablets should begin when it has been established that the supine diastolic blood pressure has begun to rise. The recommended initial dose is 200 mg, followed in 6 to 12 hours by an additional dose of 200 or 400 mg, depending on the blood pressure response. Thereafter, inpatient titration with labetalol HCl tablets may proceed as follows: Inpatient Titration Instructions Regimen Daily Dose If needed, the total daily dose may be given in three divided doses. 200 mg b.i.d. 400 mg 400 mg b.i.d. 800 mg 800 mg b.i.d. 1,600 mg 1,200 mg b.i.d. 2,400 mg While in the hospital, the dosage of labetalol HCl tablets may be increased at 1 day intervals to achieve the desired blood pressure reduction. For subsequent outpatient titration or maintenance dosing see Labetalol HCl Tablet Product Information DOSAGE AND ADMINISTRATION for additional recommendations. Compatibility with Commonly Used Intravenous Fluids Parenteral drug products should be inspected visually for particulate matter and discoloration before administration whenever solution and container permit. Labetalol HCl injection was tested for compatibility with commonly used intravenous fluids at final concentrations of 1.25 mg to 3.75 mg of labetalol HCl per milliliter of the mixture. Labetalol HCl injection was found to be compatible with and stable (for 24 hours refrigerated or at room temperature) in mixtures with the following solutions: Ringer’s Injection Lactated Ringer’s Injection 5% Dextrose and Ringer’s Injection 5% Lactated Ringer’s and 5% Dextrose Injection 5% Dextrose Injection 0.9% Sodium Chloride Injection 5% Dextrose and 0.2% Sodium Chloride Injection 2.5% Dextrose and 0.45% Sodium Chloride Injection 5% Dextrose and 0.9% Sodium Chloride Injection 5% Dextrose and 0.33% Sodium Chloride Injection. Labetalol HCl injection was NOT compatible with 5% sodium bicarbonate injection. Care should be taken when administering alkaline drugs, including furosemide, in combination with labetalol. Compatibility should be assured prior to administering these drugs together.
