Drug Catalog - Product Detail
LABETALOL HCL 300MG TB 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 49884-0124-01 | ENDO USA | 100 | 300MG | TABLET |
PACKAGE FILES

Generic Name
LABETALOL HYDROCHLORIDE
Substance Name
LABETALOL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA200908
Description
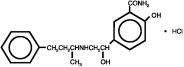
DESCRIPTION Labetalol hydrochloride tablets, USP are adrenergic receptor blocking agents that have both selective alpha 1 -adrenergic and nonselective beta-adrenergic receptor blocking actions in a single substance. Labetalol hydrochloride (HCl) is a racemate chemically designated as 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl) amino] ethyl] benzamide monohydrochloride, and it has the following structure: Labetalol HCl has the molecular formula C 19 H 24 N 2 O 3 •HCl and a molecular weight of 364.9. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R, R´stereoisomer, makes up 25% of racemic labetalol. Labetalol HCl is a white or off-white crystalline powder, soluble in water. Labetalol hydrochloride tablets contain 100, 200, or 300 mg of labetalol HCl and are taken orally. The tablets also contain the inactive ingredients lactose monohydrate, corn starch, crospovidone, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, polyethylene glycol, titanium dioxide and talc. labetalol-struc
How Supplied
HOW SUPPLIED Labetalol hydrochloride tablets, USP 100 mg, white, round, film-coated tablets with bisect, debossed “N” on top and “T” on bottom of the bisect on one side and "041" on the other side of the tablet, bottles of 100 (NDC 49884-122-01) and 500 (NDC 49884-122-05). Labetalol hydrochloride tablets, USP 200 mg, white, round, film-coated tablets with bisect, debossed “N” on top and “T” on bottom of the bisect on one side and "042" on the other side of the tablet, bottles of 100 (NDC 49884-123-01) and 500 (NDC 49884-123-05). Labetalol hydrochloride tablets, USP 300 mg, white, round, film-coated tablets with bisect, debossed “N” on top and “T” on bottom of the bisect on one side and "043" on the other side of the tablet, bottles of 100 (NDC 49884-124-01) and 500 (NDC 49884-124-05). Labetalol hydrochloride tablets, USP should be stored at 20° to 25°C (68° to 77°F)[ See USP Controlled Room Temperature]. Manufactured for: Endo USA Malvern, PA 19355 U.S.A. Made in India Neutral Code: TN/DRUGS/TN00002121 © 2024 Endo, Inc. or one of its affiliates. Revised: 08/2024
Indications & Usage
INDICATIONS AND USAGE Labetalol hydrochloride tablets, USP are indicated in the management of hypertension. Labetalol hydrochloride tablets, USP may be used alone or in combination with other antihypertensive agents, especially thiazide and loop diuretics.
Dosage and Administration
DOSAGE AND ADMINISTRATION DOSAGE MUST BE INDIVIDUALIZED. The recommended initial dosage is 100 mg twice daily whether used alone or added to a diuretic regimen. After 2 or 3 days, using standing blood pressure as an indicator, dosage may be titrated in increments of 100 mg b.i.d. every 2 or 3 days. The usual maintenance dosage of labetalol HCl is between 200 and 400 mg twice daily. Since the full antihypertensive effect of labetalol HCl is usually seen within the first 1 to 3 hours of the initial dose or dose increment, the assurance of a lack of an exaggerated hypotensive response can be clinically established in the office setting. The antihypertensive effects of continued dosing can be measured at subsequent visits, approximately 12 hours after a dose, to determine whether further titration is necessary. Patients with severe hypertension may require from 1,200 to 2,400 mg per day, with or without thiazide diuretics. Should side effects (principally nausea or dizziness) occur with these doses administered twice daily, the same total daily dose administered three times daily may improve tolerability and facilitate further titration. Titration increments should not exceed 200 mg twice daily. When a diuretic is added, an additive antihypertensive effect can be expected. In some cases this may necessitate a labetalol HCl dosage adjustment. As with most antihypertensive drugs, optimal dosages of labetalol hydrochloride tablets are usually lower in patients also receiving a diuretic. When transferring patients from other antihypertensive drugs, labetalol hydrochloride tablets should be introduced as recommended and the dosage of the existing therapy progressively decreased. Elderly Patients: As in the general patient population, labetalol therapy may be initiated at 100 mg twice daily and titrated upwards in increments of 100 mg b.i.d. as required for control of blood pressure. Since some elderly patients eliminate labetalol more slowly, however, adequate control of blood pressure may be achieved at a lower maintenance dosage compared to the general population. The majority of elderly patients will require between 100 and 200 mg b.i.d.
