Drug Catalog - Product Detail
KETOCONAZOLE FOAM FOAM 0.02 50GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0532-32 | PADAGIS | 50 | 2% | FOAM |
PACKAGE FILES


Generic Name
KETOCONAZOLE
Substance Name
KETOCONAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA091550
Description
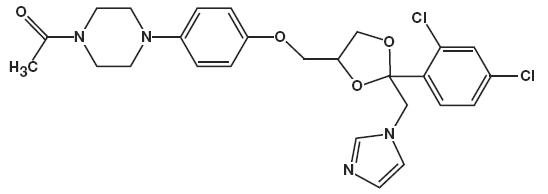
11 DESCRIPTION Ketoconazole foam, 2% contains 2% ketoconazole USP, an antifungal agent, in a thermolabile hydroethanolic foam for topical application. The chemical name for ketoconazole is piperazine, 1-acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2-(1 H -imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-, cis - with the molecular formula C 26 H 28 Cl 2 N 4 O 4 and a molecular weight of 531.43. The following is the chemical structure: Ketoconazole foam, 2% contains 20 mg ketoconazole per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol, citric acid, ethanol 58%, polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant. chemical structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Ketoconazole Foam, 2% contains 20 mg of ketoconazole, USP per gram. The thermolabile hydroethanolic foam is available as follows: NDC 45802-532-32 50 g aluminum can NDC 45802-532-33 100 g aluminum can Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Do not store under refrigerated conditions. Contents are flammable. Do not expose containers to heat and/or store at temperatures above 49°C (120°F). Do not store in direct sunlight. Contents under pressure. Do not puncture and/or incinerate container. Keep out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Ketoconazole foam, 2% is indicated for the topical treatment of seborrheic dermatitis in immunocompetent patients 12 years of age and older. Limitations of Use Safety and efficacy of ketoconazole foam, 2% for treatment of fungal infections have not been established. Ketoconazole foam, 2% is indicated for topical treatment of seborrheic dermatitis in immunocompetent patients 12 years of age and older. Limitations of Use Safety and efficacy of ketoconazole foam, 2% for treatment of fungal infections have not been established.
Dosage and Administration
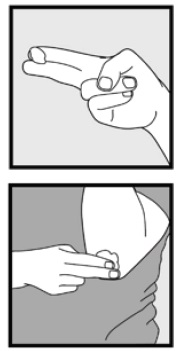
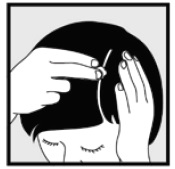
2 DOSAGE AND ADMINISTRATION Ketoconazole foam, 2% should be applied to the affected area(s) twice daily for four weeks. Hold the container upright, and dispense ketoconazole foam, 2% into the cap of the can or other cool surface in an amount sufficient to cover the affected area(s). Dispensing directly onto hands is not recommended, as the foam will begin to melt immediately upon contact with warm skin. Pick up small amounts of ketoconazole foam, 2% with the fingertips, and gently massage into the affected area(s) until the foam disappears. For hair-bearing areas, part the hair, so that ketoconazole foam, 2% may be applied directly to the skin (rather than on the hair). Avoid contact with the eyes and other mucous membranes. Ketoconazole foam, 2% is not for ophthalmic, oral or intravaginal use. • Ketoconazole foam, 2% should be applied to the affected area(s) twice daily for four weeks (2). • Ketoconazole foam, 2% is not for ophthalmic, oral, or intravaginal use (2).
