Drug Catalog - Product Detail
ISOTRETINOIN (MYORISAN) 30MG CAP 3X10
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61748-0303-13 | AKORN | 30 | 30MG | CAPSULE |
PACKAGE FILES





Generic Name
Substance Name
Product Type
Route
Application Number
Description
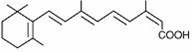
DESCRIPTION Isotretinoin, a retinoid, is available as Myorisan ® in 10-mg, 20-mg, 30-mg and 40-mg soft gelatin capsules for oral administration. Each capsule contains yellow wax, butylated hydroxyanisole, edetate disodium, hydrogenated vegetable oil, tocopherol, and soybean oil. Gelatin capsules contain gelatin, glycerin and non-crystallizing sorbitol solution, with the following dye systems: 10 mg — ferric oxide (yellow) and titanium dioxide; 20 mg — titanium dioxide; 30 mg — titanium dioxide and ferric oxide (red); 40 mg — FD&C Yellow No.6 and titanium dioxide. The edible imprinting ink for all the capsules contains: shellac glaze, dehydrated alcohol, isopropyl alcohol, iron oxide black, N-butyl alcohol, propylene glycol, and ammonium hydroxide. Meets USP Dissolution Test 6. Chemically, isotretinoin is 13-cis-retinoic acid and is related to both retinoic acid and retinol (vitamin A). It is a yellow to orange crystalline powder with a molecular weight of 300.44. The structural formula is: Chemical Structure
How Supplied
HOW SUPPLIED Soft gelatin capsules, 10 mg (pale yellow), imprinted in black ink with “V10”. Boxes of 30 containing 3 Prescription Packs of 10 capsules (NDC 61748-301-13). Boxes of 100 containing 10 Prescription Packs of 10 capsules (NDC 61748-301-11). Soft gelatin capsules, 20 mg (white to slight pink), imprinted in black ink with “V20”. Boxes of 30 containing 3 Prescription Packs of 10 capsules (NDC 61748-302-13). Boxes of 100 containing 10 Prescription Packs of 10 capsules (NDC 61748-302-11). Soft gelatin capsules, 30 mg (pink), imprinted in black ink with “V30”. Boxes of 30 containing 3 Prescription Packs of 10 capsules (NDC 61748-303-13). Boxes of 100 containing 10 Prescription Packs of 10 capsules (NDC 61748-303-11). Soft gelatin capsules, 40 mg (orange), imprinted in black ink with “V40”. Boxes of 30 containing 3 Prescription Packs of 10 capsules (NDC 61748-304-13). Boxes of 100 containing 10 Prescription Packs of 10 capsules (NDC 61748-304-11). Storage Store at 20°-25°C (68° to 77°F). [See USP controlled room temperature]. Protect from light.
Indications & Usage
INDICATIONS AND USAGE Severe Recalcitrant Nodular Acne Myorisan ® is indicated for the treatment of severe recalcitrant nodular acne. Nodules are inflammatory lesions with a diameter of 5 mm or greater. The nodules may become suppurative or hemorrhagic. “Severe,” by definition, 2 means “many” as opposed to “few or several” nodules. Because of significant adverse effects associated with its use, Myorisan ® should be reserved for patients with severe nodular acne who are unresponsive to conventional therapy, including systemic antibiotics. In addition, Myorisan ® is indicated only for those patients who are not pregnant, because Myorisan ® can cause life-threatening birth defects (see Boxed CONTRAINDICATIONS AND WARNINGS ). A single course of therapy for 15 to 20 weeks has been shown to result in complete and prolonged remission of disease in many patients. 1,3,4 If a second course of therapy is needed, it should not be initiated until at least 8 weeks after completion of the first course, because experience has shown that patients may continue to improve while off Myorisan ® . The optimal interval before retreatment has not been defined for patients who have not completed skeletal growth (see WARNINGS: Skeletal: Bone Mineral Density , Hyperostosis , Premature Epiphyseal Closure ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Myorisan ® should be administered with a meal (see PRECAUTIONS : Information for Patients ). The recommended dosage range for Myorisan ® is 0.5 to 1 mg/kg/day given in two divided doses with food for 15 to 20 weeks. In studies comparing 0.1, 0.5, and 1 mg/kg/day, 8 it was found that all dosages provided initial clearing of disease, but there was a greater need for retreatment with the lower dosages. During treatment, the dose may be adjusted according to response of the disease and/or the appearance of clinical side effects — some of which may be dose related. Adult patients whose disease is very severe with scarring or is primarily manifested on the trunk may require dose adjustments up to 2 mg/kg/day, as tolerated. Failure to take Myorisan ® with food will significantly decrease absorption. Before upward dose adjustments are made, the patients should be questioned about their compliance with food instructions. The safety of once daily dosing with Myorisan ® has not been established. Once daily dosing is not recommended. If the total nodule count has been reduced by more than 70% prior to completing 15 to 20 weeks of treatment, the drug may be discontinued. After a period of 2 months or more off therapy, and if warranted by persistent or recurring severe nodular acne, a second course of therapy may be initiated. The optimal interval before retreatment has not been defined for patients who have not completed skeletal growth. Long-term use of Myorisan ® , even in low doses, has not been studied, and is not recommended. It is important that Myorisan ® be given at the recommended doses for no longer than the recommended duration. The effect of long-term use of Myorisan ® on bone loss is unknown (see WARNINGS: Skeletal: Bone Mineral Density , Hyperostosis , and Premature Epiphyseal Closure ). Contraceptive measures must be followed for any subsequent course of therapy (see PRECAUTIONS ). Table 4 Myorisan ® Dosing by Body Weight (Based on Administration With Food) Body Weight Total mg/day kilograms pounds 0.5 mg/kg 1 mg/kg 2 mg/kg See DOSAGE AND ADMINISTRATION : the recommended dosage range is 0.5 to 1 mg/kg/day. 40 88 20 40 80 50 110 25 50 100 60 132 30 60 120 70 154 35 70 140 80 176 40 80 160 90 198 45 90 180 100 220 50 100 200 INFORMATION FOR PHARMACISTS Access the iPLEDGE REMS system via the internet ( www.ipledgeprogram.com ), telephone (1-866-495-0654) to obtain an authorization and the "do not dispense to patient after" date. Myorisan ® must only be dispensed in no more than a 30-day supply. REFILLS REQUIRE A NEW PRESCRIPTION AND A NEW AUTHORIZATION FROM THE iPLEDGE SYSTEM. A Myorisan ® Medication Guide must be given to the patient each time Myorisan ® is dispensed, as required by law. This Myorisan ® Medication Guide is an important part of the risk management program for the patient.
