Drug Catalog - Product Detail
Isosorbide Dinitrate Tab 5 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68682-0193-01 | OCEANSIDE PHARMACEUTICALS | 100 | 5MG | NA |
PACKAGE FILES


Generic Name
ISOSORBIDE DINITRATE
Substance Name
ISOSORBIDE DINITRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA012093
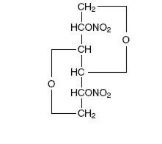
Description
DESCRIPTION Isosorbide dinitrate (ISDN) is 1,4:3,6-dianhydro-D-glucitol 2,5-dinitrate, an organic nitrate whose structural formula is and whose molecular weight is 236.14. The organic nitrates are vasodilators, active on both arteries and veins. Isosorbide dinitrate is a white, crystalline, odorless compound which is stable in air and in solution, has a melting point of 70°C and has an optical rotation of +134° (c=1.0, alcohol, 20°C). Isosorbide dinitrate is freely soluble in organic solvents such as acetone, alcohol, and ether, but is only sparingly soluble in water. Each Isosorbide Dinitrate tablet contains 5 mg or 40 mg of isosorbide dinitrate. The inactive ingredients in 5 mg tablet are magnesium stearate, lactose monohydrate, microcrystalline cellulose and FD&C red 40. The inactive ingredients in 40 mg tablet are magnesium stearate, microcrystalline cellulose, FD&C yellow 6, D&C yellow 10, and FD&C blue. Chemical Structure
How Supplied
HOW SUPPLIED Isosorbide Dinitrate tablets are available as follows: 5 mg, round, pink tablets imprinted "BPI 152" on one side and deeply scored on reverse side: NDC 68682-193-01, bottles of 100. 40 mg, round, light green tablets imprinted "BPI 192" on one side and deeply scored on reverse side: NDC 68682-192-01, bottles of 100. Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light. Keep bottles tightly closed. Dispense in a light-resistant, tight container. Keep out of reach of children Distributed by: Oceanside Pharmaceuticals, a division of Bausch Health US, LLC Bridgewater, NJ 08807 USA Manufactured by: Bausch Health Companies Inc. Steinbach, MB R5G 1Z7, Canada © 2020 Bausch Health Companies Inc. or its affiliates 9704300 20002849 Rev. 03/2020
Indications & Usage
INDICATIONS AND USAGE Isosorbide Dinitrate tablets are indicated for the prevention of angina pectoris due to coronary artery disease. The onset of action of immediate-release oral isosorbide dinitrate is not sufficiently rapid for this product to be useful in aborting an acute anginal episode.
Dosage and Administration
DOSAGE AND ADMINISTRATION As noted under CLINICAL PHARMACOLOGY , multiple-dose studies with ISDN and other nitrates have shown that maintenance of continuous 24-hour plasma levels results in refractory tolerance. Every dosing regimen for Isosorbide Dinitrate tablets must provide a daily dose-free interval to minimize the development of this tolerance. With immediate-release ISDN, it appears that one daily dose-free interval must be at least 14 hours long. As also noted under CLINICAL PHARMACOLOGY , the effects of the second and later doses have been smaller and shorter-lasting than the effects of the first. Large controlled studies with other nitrates suggest that no dosing regimen with Isosorbide Dinitrate tablets should be expected to provide more than about 12 hours of continuous anti-anginal efficacy per day. As with all titratable drugs, it is important to administer the minimum dose which produces the desired clinical effect. The usual starting dose of Isosorbide Dinitrate is 5 mg to 20 mg two or three times daily. For maintenance therapy, 10 mg to 40 mg two or three times daily is recommended. Some patients may require higher doses. A daily dose-free interval of at least 14 hours is advisable to minimize tolerance. The optimal interval will vary with the individual patient, dose and regimen.
