Drug Catalog - Product Detail
IRBESARTAN/HYDROCHLOROTHIAZIDE TAB 12.5MG/150MG 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43547-0330-09 | SOLCO HEALTHCARE | 90 | 150-12.5MG | TABLET |
PACKAGE FILES


Generic Name
IRBESARTAN AND HYDROCHLOROTHIAZIDE
Substance Name
HYDROCHLOROTHIAZIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203072
Description
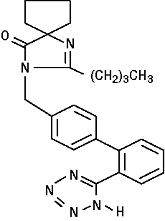
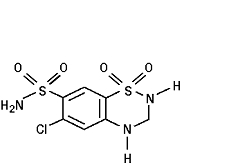
11 DESCRIPTION Irbesartan and hydrochlorothiazide tablets are a combination of an angiotensin II receptor antagonist (AT 1 subtype), irbesartan, and a thiazide diuretic, hydrochlorothiazide (HCTZ). Irbesartan is a non-peptide compound, chemically described as a 2-butyl-3-[ p -( o -1 H -tetrazol-5-ylphenyl)benzyl]-1,3-diazaspiro[4.4]non-1-en-4-one. Its empirical formula is C 25 H 28 N 6 O, and its structural formula is: Irbesartan, USP, is a white to off-white crystalline powder with a molecular weight of 428.5. It is a nonpolar compound with a partition coefficient (octanol/water) of 10.1 at pH of 7.4. Irbesartan is slightly soluble in alcohol and methylene chloride and practically insoluble in water. Hydrochlorothiazide is 6-chloro-3,4-dihydro-2 H -1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C 7 H 8 ClN 3 O 4 S 2 and its structural formula is: Hydrochlorothiazide, USP, is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water and freely soluble in sodium hydroxide solution. Irbesartan and hydrochlorothiazide tablets, USP, are available for oral administration in film-coated tablets containing either 150 mg or 300 mg of irbesartan combined with 12.5 mg of hydrochlorothiazide. All dosage strengths contain the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, hypromellose, magnesium stearate, polyvinyl alcohol-part.hydrolyzed, titanium dioxide, talc, macrogol/PEG 3350, lecithin, ferric oxide red, ferric oxide yellow, ferric oxide black. Chemical Structure Irbesartan Chemical Structure Hydrochlorothiazide
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Irbesartan and Hydrochlorothiazide film-coated tablets have markings on both sides and are available in the strengths and packages listed in the following table: Tablet Strength (irbesartan and hydrochlorothiazide) Film-Coated Tablet Color/Shape Tablet Markings Package Size NDC Code 150 mg and 12.5 mg Pink, oval-shaped Debossed '325' on one side and 'HH' on the other side Bottles of 30 43547-330-03 Bottles of 90 43547-330-09 Bottles of 500 43547-330-50 300 mg and 12.5 mg Pink, capsule-shaped Debossed '327' on one side and 'HH' on the other side Bottles of 30 43547-331-03 Bottles of 90 43547-331-09 Bottles of 500 43547-331-50 16.2 Storage Store at 20°C-25°C (68°F-77°F); excursions permitted to 15°C-30°C (59°F-86°F) [see USP Controlled Room Temperature].
Indications & Usage
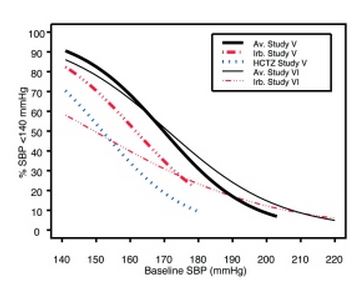
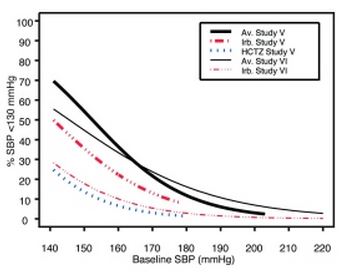
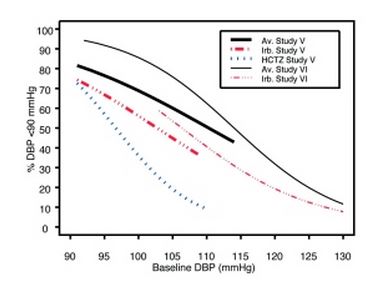
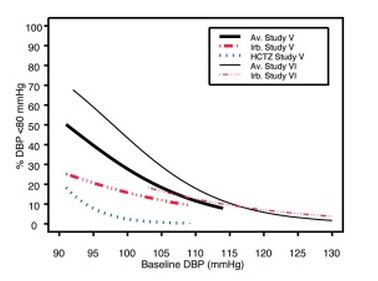
1 INDICATIONS AND USAGE Irbesartan and hydrochlorothiazide tablets are indicated for the treatment of hypertension. Irbesartan and hydrochlorothiazide tablets may be used in patients whose blood pressure is not adequately controlled on monotherapy. Irbesartan and hydrochlorothiazide tablets may also be used as initial therapy in patients who are likely to need multiple drugs to achieve their blood pressure goals. The choice of irbesartan and hydrochlorothiazide tablets as initial therapy for hypertension should be based on an assessment of potential benefits and risks. Patients with stage 2 (moderate or severe) hypertension are at relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and may be shaped by considerations such as the baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared with monotherapy. Data from Studies V and VI [see Clinical Studies (14.2) ] provide estimates of the probability of reaching a blood pressure goal with irbesartan and hydrochlorothiazide tablets compared to irbesartan or hydrochlorothiazide (HCTZ) monotherapy. The relationship between baseline blood pressure and achievement of a SeSBP <140 or <130 mmHg or SeDBP <90 or <80 mmHg in patients treated with irbesartan and hydrochlorothiazide tablets compared to patients treated with irbesartan or HCTZ monotherapy are shown in Figures 1a through 2b. Figure 1a: Probability of Achieving SBP <140 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * Figure 1b: Probability of Achieving SBP <130 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * Figure 2a: Probability of Achieving DBP <90 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * Figure 2b: Probability of Achieving DBP <80 mmHg in Patients from Initial Therapy Studies V (Week 8) and VI (Week 7) * The above graphs provide a rough approximation of the likelihood of reaching a targeted blood pressure goal (e.g., Week 8 sitting systolic blood pressure ≤140 mmHg) for the treatment groups. The curve of each treatment group in each study was estimated by logistic regression modeling from all available data of that treatment group. The estimated likelihood at the right tail of each curve is less reliable due to small numbers of subjects with high baseline blood pressures. For example, a patient with a blood pressure of 180/105 mmHg has about a 25% likelihood of achieving a goal of <140 mmHg (systolic) and 50% likelihood of achieving <90 mmHg (diastolic) on irbesartan alone (and lower still likelihoods on HCTZ alone). The likelihood of achieving these goals on irbesartan and hydrochlorothiazide tablets rise to about 40% (systolic) or 70% (diastolic). Irbesartan and hydrochlorothiazide tablets are a combination of irbesartan, an angiotensin II receptor antagonist, and hydrochlorothiazide, a thiazide diuretic, indicated for hypertension: • In patients not adequately controlled with monotherapy. (1) • As initial therapy in patients likely to need multiple drugs to achieve their blood pressure goals. (1) Irbesartan and Hydrochlorothiazide Figure 1A Irbesartan and Hydrochlorothiazide Figure 1B Irbesartan and Hydrochlorothiazide Figure 2A Irbesartan and Hydrochlorothiazide Figure 2B
Dosage and Administration
2 DOSAGE AND ADMINISTRATION General Considerations • Maximum effects within 2 to 4 weeks after dose change. (2.1) • Renal impairment: Not recommended for patients with severe renal impairment (creatinine clearance <30 mL/min). (2.1 , 5.8) Hypertension • Initiate with 150/12.5 mg. Titrate to 300/12.5 mg then 300/25 mg if needed. (2.2) • Replacement therapy: May be substituted for titrated components. (2.3) 2.1 General Considerations The side effects of irbesartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. [See Adverse Reactions (6) .] Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose. Irbesartan and hydrochlorothiazide tablets may be administered with or without food. Irbesartan and hydrochlorothiazide tablets may be administered with other antihypertensive agents. Renal Impairment The usual regimens of therapy with irbesartan and hydrochlorothiazide tablets may be followed as long as the patient’s creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so irbesartan and hydrochlorothiazide tablets are not recommended. Hepatic Impairment No dosage adjustment is necessary in patients with hepatic impairment. 2.2 Add-On Therapy In patients not controlled on monotherapy with irbesartan or hydrochlorothiazide, the recommended doses of irbesartan and hydrochlorothiazide tablets, in order of increasing mean effect, are (irbesartan and hydrochlorothiazide) 150/12.5 mg, 300/12.5 mg, and 300/25 mg. The largest incremental effect will likely be in the transition from monotherapy to 150/12.5 mg. [See Clinical Studies (14.2) .] 2.3 Replacement Therapy Irbesartan and hydrochlorothiazide tablets may be substituted for the titrated components. 2.4 Initial Therapy The usual starting dose is irbesartan and hydrochlorothiazide tablets 150/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of 300/25 mg once daily as needed to control blood pressure [see Clinical Studies (14.2) ]. Irbesartan and hydrochlorothiazide tablets are not recommended as initial therapy in patients with intravascular volume depletion [see Warnings and Precautions (5.2) ].
