Drug Catalog - Product Detail
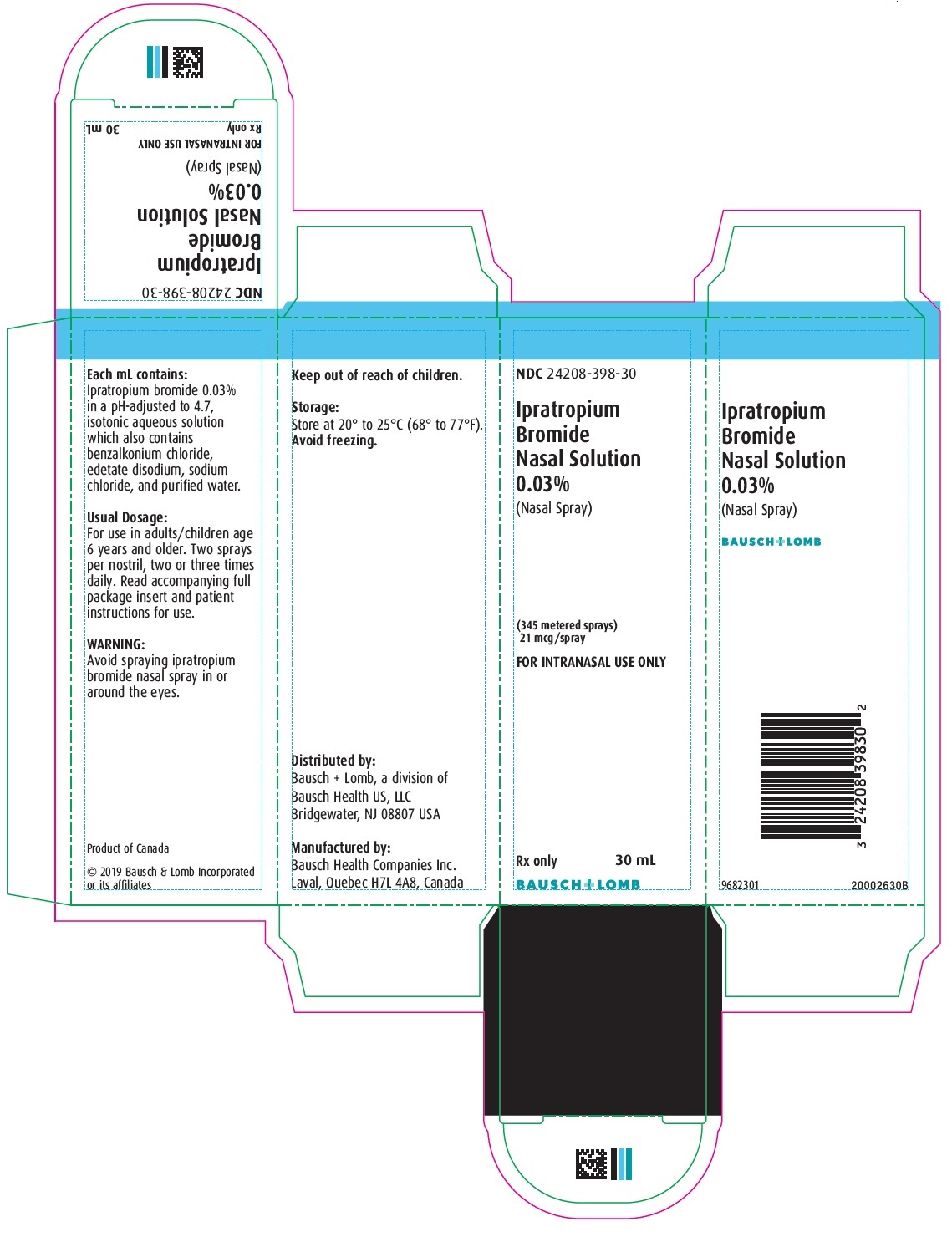
IPRATROPIUM BROMIDE INHAL SOL. SPR 0.0003 30ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0398-30 | BAUSCH HEALTH US | 30 | 0.03% | SOLUTION |
PACKAGE FILES
Generic Name
IPRATROPIUM BROMIDE
Substance Name
IPRATROPIUM BROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
NASAL
Application Number
ANDA076025
Description
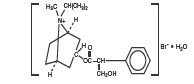
DESCRIPTION: The active ingredient in Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray) is ipratropium bromide monohydrate. It is an anticholinergic agent chemically described as 8-azoniabicyclo[3.2.1] octane, 3-(3-hydroxy-1-oxo-2 phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide monohydrate (3-endo, 8-syn)-: a synthetic quaternary ammonium compound, chemically related to atropine. Its structural formula is: ipratropium bromide monohydrate C 20 H 30 BrNO 3 • H 2 O Mol. Wt. 430.4 Ipratropium bromide is a white to off-white crystalline substance, freely soluble in water and methanol, sparingly soluble in ethanol, and insoluble in non-polar media. In aqueous solution, it exists in an ionized state as a quaternary ammonium compound. Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray) is a metered-dose, manual pump spray unit which delivers 21 mcg (70 microliters) ipratropium bromide per spray on an anhydrous basis in an isotonic, aqueous solution, with pH-adjusted to 4.7 with hydrochloric acid and/or sodium hydroxide (if needed). It also contains benzalkonium chloride, edetate disodium, sodium chloride and purified water. Each bottle contains 345 sprays. ipratropium bromide monohydrate (structural formula)
How Supplied
HOW SUPPLIED: Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray) is supplied as 30 mL of solution in a high density polyethylene (HDPE) bottle fitted with a metered nasal spray pump, a safety clip to prevent accidental discharge of the spray, and a plastic dust cap. It contains 31.1 g of product formulation, 345 sprays, each delivering 21 mcg (70 microliters) of ipratropium per spray, or 28 days of therapy at the maximum recommended dose (two sprays per nostril three times a day). NDC 24208-398-30 Bottle of 30 mL
Indications & Usage
INDICATIONS AND USAGE: Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray) is indicated for the symptomatic relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray) does not relieve nasal congestion, sneezing, or postnasal drip associated with allergic or nonallergic perennial rhinitis.
Dosage and Administration
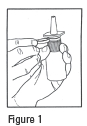
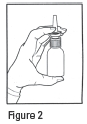
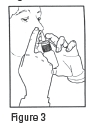
DOSAGE AND ADMINISTRATION: The recommended dose of Ipratropium Bromide Nasal Solution 0.03% (Nasal Spray) is two sprays (42 mcg) per nostril two or three times daily (total dose 168 to 252 mcg/day) for the symptomatic relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. Optimum dosage varies with the response of the individual patient. Initial pump priming requires seven sprays of the pump. If used regularly as recommended, no further priming is required. If not used for more than 24 hours, the pump will require two sprays, or if not used for more than seven days, the pump will require seven sprays to reprime. Avoid spraying into eyes .
