Drug Catalog - Product Detail
IPRATROPIUM BROMIDE INHAL SOL. 0.02% 30X2.5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00487-9801-30 | NEPHRON PHARMACEUTICALS CORP. | 2 | 0.02% | SOLUTION |
PACKAGE FILES






Generic Name
IPRATROPIUM BROMIDE
Substance Name
IPRATROPIUM BROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
RESPIRATORY (INHALATION)
Application Number
ANDA075562
Description
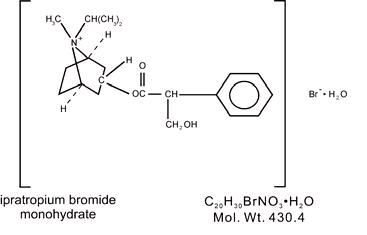
DESCRIPTION The active ingredient in ipratropium bromide inhalation solution is ipratropium bromide monohydrate. It is an anticholinergic bronchodilator chemically described as 8-Azoniabicyclo [3.2.1]-octane,-3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate ( endo, syn )-,(±)-; a synthetic quaternary ammonium compound, chemically related to atropine. Ipratropium bromide is a white crystalline substance, freely soluble in water and lower alcohols. It is a quaternary ammonium compound and thus exists in an ionized state in aqueous solutions. It is relatively insoluble in non-polar media. Ipratropium bromide inhalation solution USP is administered by oral inhalation with the aid of a nebulizer. Each mL contains ipratropium bromide USP 0.02% (anhydrous basis) in a sterile, preservative-free, isotonic saline solution, pH-adjusted to 3.4 (3 to 4) with hydrochloric acid. 97e130d5-figure-01
How Supplied
HOW SUPPLIED Ipratropium bromide inhalation solution USP is a clear, colorless solution containing 2.5 mL, packaged in cartons as listed below: NDC 0487-9801-25, 25 vials per carton / 5 vials per foil pouch NDC 0487-9801-30, 30 vials per carton / 5 vials per foil pouch NDC 0487-9801-60, 60 vials per carton / 5 vials per foil pouch NDC 0487-9801-01, 30 vials per carton / 1 vial per foil pouch Each vial is made from a low density polyethylene (LDPE) resin. Storage and Handling Store between 15° C and 30° C (59° F and 86° F). Protect from light. Store in pouch until time of use. ATTENTION PHARMACIST: Detach “Patient’s Information for Use” from Package Insert and dispense with solution. Manufactured By: Nephron Pharmaceuticals Corporation West Columbia, SC 29172 For Customer Service, Call 1-800-443-4313
Indications & Usage
INDICATIONS AND USAGE Ipratropium bromide inhalation solution administered either alone or with other bronchodilators, especially beta adrenergics, is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema.
Dosage and Administration
DOSAGE AND ADMINISTRATION The usual dosage of ipratropium bromide inhalation solution is 500 mcg (1 Unit-Dose Vial) administered three to four times a day by oral nebulization, with doses 6 to 8 hours apart. Ipratropium bromide inhalation solution Unit-Dose Vials contain 500 mcg ipratropium bromide anhydrous in 2.5 mL normal saline. Ipratropium bromide inhalation solution can be mixed in the nebulizer with albuterol or metaproterenol if used within one hour. Drug stability and safety of ipratropium bromide inhalation solution when mixed with other drugs in a nebulizer have not been established.
