Drug Catalog - Product Detail
IMIQUIMOD CREAM CRM 0.05 BOX-24PCKT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0536-70 | GLENMARK PHARMACEUTICALS | 24 | 5% | CREAM |
PACKAGE FILES



Generic Name
IMIQUIMOD
Substance Name
IMIQUIMOD
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA201994
Description
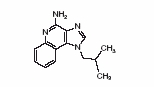
11 DESCRIPTION Imiquimod cream USP is an immune response modifier for topical administration. Each gram contains 50 mg of imiquimod, USP in an off-white oil-in-water vanishing cream base consisting of benzyl alcohol, cetyl alcohol, glycerin, isostearic acid, methylparaben, polysorbate 60, propylparaben, purified water, sorbitan monostearate, stearyl alcohol, white petrolatum and xanthan gum. Chemically, imiquimod, USP is 1-(2-methylpropyl)-1 H-imidazo[4,5-c]quinolin-4-amine. Imiquimod, USP has a molecular formula of C 14 H 16 N 4 and a molecular weight of 240.3 g/mol. Its structural formula is: structure.jpg
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Imiquimod Cream USP, 5% is supplied in single-use packets, which contains 250 mg of the cream. Available as: Carton (NDC 68462-536-70) of 24 packets (NDC 68462-536-01). Store at 4°C to 25°C (39°F to 77°F). Avoid freezing. Keep out of reach of children.
Indications & Usage
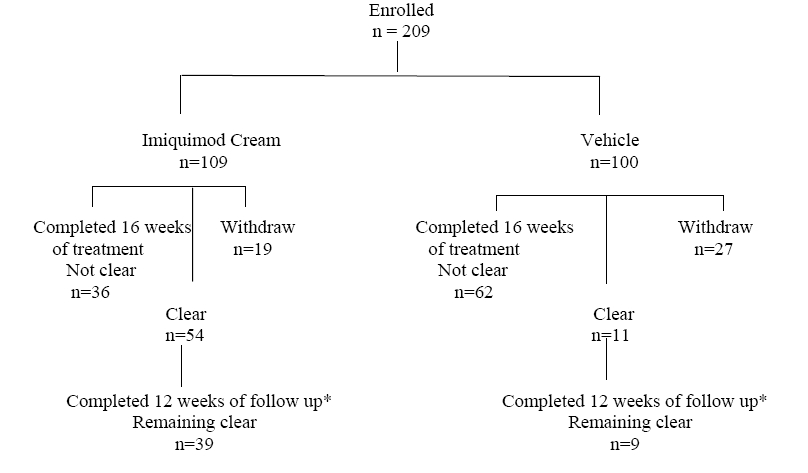
1 INDICATIONS AND USAGE Imiquimod cream is indicated for the topical treatment of: • Clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses (AK) on the face or scalp in immunocompetent adults. ( 1.1 ) • Biopsy-confirmed, primary superficial basal cell carcinoma (sBCC) in immunocompetent adults with a maximum tumor diameter of 2.0 cm on trunk (excluding anogenital skin), neck, or extremities (excluding hands and feet), only when surgical methods are medically less appropriate and patient follow-up can be reasonably assured. ( 1.2 ) • External genital and perianal warts (EGW) in immunocompetent patients 12 years of age and older. ( 1.3 ) 1.1 Actinic Keratosis Imiquimod Cream is indicated for the topical treatment of clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratosis (AK) on the face or scalp in immunocompetent adults. 1.2 Superficial Basal Cell Carcinoma Imiquimod Cream is indicated for the topical treatment of biopsy-confirmed, primary superficial basal cell carcinoma (sBCC) in immunocompetent adults, with a maximum tumor diameter of 2.0 cm, located on the trunk (excluding anogenital skin), neck, or extremities (excluding hands and feet), only when surgical methods are medically less appropriate and patient follow-up can be reasonably assured. Establish the histological diagnosis of superficial basal cell carcinoma prior to treatment. The safety and effectiveness of Imiquimod Cream have not been established for other types of basal cell carcinomas (BCC), including nodular and morpheaform (fibrosing or sclerosing) types. 1.3 External Genital Warts Imiquimod Cream is indicated for the topical treatment of external genital and perianal warts (EGW) in immunocompetent patients 12 years of age and older.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • For topical use only; not for oral, ophthalmic, intra-anal or intravaginal use. ( 2.1 ) • AK : Apply once daily before bedtime 2 times per week for a full 16 weeks to a contiguous area of approximately 25 cm 2 on the face or scalp. Apply no more than 1 packet at each application. ( 2.2 ) • sBCC : Apply once daily before bedtime 5 times per week for a full 6 weeks to a target tumor with 2 cm maximum diameter on the trunk (excluding anogenital skin), neck, or extremities (excluding hands and feet). Amount of Imiquimod Cream used based on target tumor diameter. ( 2.3 ) • EGW: Apply thin layer once daily before bedtime 3 times per week until total clearance or for a maximum of 16 weeks. ( 2.4 ) 2.1 Important Dosage and Administration Instructions Imiquimod Cream is for topical use only. Imiquimod Cream is not for oral, ophthalmic, or intravaginal use. Instruct patients on proper application technique. Wash hands before and after applying Imiquimod Cream. Wash the treatment area with mild soap and water and allow the area to dry thoroughly (at least 10 minutes) before applying Imiquimod Cream. If an Imiquimod Cream dose is missed, apply the next dose at the regularly scheduled time. Avoid contact with the eyes, lips, nostrils, or inside the anus and vagina. For patients with AK and sBCC, prescribe no more than 3 carton (36 packets) of Imiquimod Cream for the entire treatment period. For EGW, one packet of Imiquimod Cream contains sufficient cream to cover a wart area of up to 20 cm 2 . Discard partially used packets and do not reuse. 2.2 Dosage and Administration for Actinic Keratosis Apply Imiquimod Cream topically once daily before bedtime 2 times per week for a full 16 weeks to a defined treatment area of AK on the face or scalp (but not both concurrently). A treatment area is defined as one contiguous area of approximately 25 cm 2 (e.g., 5 cm x 5 cm) on the face (e.g., forehead or one cheek) or on the scalp. Apply Imiquimod Cream to the entire treatment area and rub in until the cream is no longer visible. Apply no more than 1 packet of Imiquimod Cream to the contiguous treatment area at each application. Leave Imiquimod Cream on the skin for approximately 8 hours and then remove with mild soap and water. For local skin reactions a dosage interruption of several days may be taken if required by the patient's discomfort or severity of the local skin reaction [see Warnings and Precautions ( 5.1 )] . Do not extend treatment beyond 16 weeks due to missed doses or rest periods. Assess response to treatment after resolution of local skin reactions. 2.3 Dosage and Administration for Superficial Basal Cell Carcinoma Apply Imiquimod Cream topically once daily before bedtime 5 times per week for a full 6 weeks to a biopsy-confirmed sBCC. The target tumor should have a maximum diameter of 2 cm and be located on the trunk (excluding anogenital skin), neck, or extremities (excluding hands and feet). The amount of cream needed to cover the target tumor, including 1 cm of skin surrounding the tumor, is presented in Table 1. Rub Imiquimod Cream into the treatment area until the cream is no longer visible. Leave Imiquimod Cream on the skin for approximately 8 hours and then remove with mild soap and water. Table 1: Amount of Imiquimod Cream to Use for sBCC Target Tumor Diameter Size of Cream Droplet to be Used (Diameter) Approximate Amount of Imiquimod Cream to be Used 0.5 to <1.0 cm 4 mm 10 mg ≥1.0 to <1.5 cm 5 mm 25 mg ≥1.5 to 2.0 cm 7 mm 40 mg For local skin reactions a dosage interruption of several days may be taken if required by the patient's discomfort or severity of the local skin reaction [see Warnings and Precautions ( 5.1 )] . Assess for early clinical clearance after resolution of local skin reactions (e.g., 12 weeks post-treatment). Local skin reactions or other findings (e.g., infection) may require that a patient be seen sooner than the post-treatment assessment for clinical clearance. If there is clinical evidence of persistent tumor at the post-treatment assessment for clinical clearance, consider a biopsy or other alternative intervention. Instruct patients to contact their healthcare provider if any suspicious lesion arises in the treatment area at any time after a determination of clinical clearance [see Clinical Studies ( 14.2 )] . 2.4 Dosage and Administration for External Genital Warts Apply a thin layer of Imiquimod Cream topically once daily before bedtime 3 times per week to EGW until there is total clearance of the genital/perianal warts or for a maximum of 16 weeks. Rub in until the cream is no longer visible. Do not occlude the application site. Leave Imiquimod Cream on the skin for 6 to 10 hours and then remove with mild soap and water. For local skin reactions, a dosage interruption of several days may be taken if required by the patient's discomfort or severity of the local skin reaction [see Warnings and Precautions ( 5.1 )] . Treatment may resume once the reaction subsides. Nonocclusive dressings such as cotton gauze or cotton underwear may be used to manage skin reactions. Inform uncircumcised patients treating warts under the foreskin to retract the foreskin and clean the area daily. Imiquimod Cream may weaken condoms and vaginal diaphragms; therefore, concurrent use is not recommended.
