Drug Catalog - Product Detail
IMIPRAMINE HCL, USP TB 10MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69315-0133-01 | LEADING PHARMA | 100 | 10MG | TABLET |
PACKAGE FILES






Generic Name
IMIPRAMINE HYDROCHLORIDE
Substance Name
IMIPRAMINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040903
Description
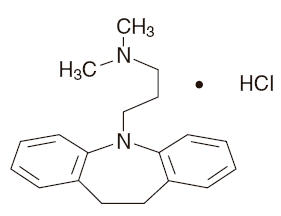
DESCRIPTION Imipramine hydrochloride tablets USP are supplied in tablet form for oral administration. Imipramine hydrochloride USP, the original tricyclic antidepressant, are a member of the dibenzazepine group of compounds. It is designated 5-3-(dimethylamino) propyl-10, 11-dihydro-5H dibenz [b,f]- azepine monohydrochloride. Its structural formula is: C19H24N2 • HCl MW = 316 .88 Imipramine hydrochloride USP is a white to off-white, odorless, or practically odorless crystalline powder. It is freely soluble in water and in alcohol, soluble in acetone, and insoluble in ether and in benzene. Inactive Ingredients: Colloidal silicon dioxide, D & C red # 30 and # 40, D & C yellow # 6 and # 10, dicalcium phosphate, F D & C blue # 1 and # 2, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate, sodium starch glycolate, titanium dioxide. Chemical Structure
How Supplied
HOW SUPPLIED The three strengths of imipramine hydrochloride tablets USP, are available as follows: Tablets 10 mg - round, yellow, compressed, film-coated tablet, debossed with "EP" and "133" on one side and plain on the other side. Bottles of 100 ............ NDC 69315-133-01 Bottles of 1000 ..........NDC 69315-133-10 Tablets 25 mg - round, brown, compressed, film-coated tablet, debossed with "EP" and "134" on one side and plain on the other side. Bottles of 100 ............ NDC 69315-134-01 Bottles of 1000 ..........NDC 69315-134-10 Tablets 50 mg - round, green, compressed, film-coated tablet, debossed with "EP" and "135" on one side and plain on the other side. Bottles of 100 ............ NDC 69315-135-01 Bottles of 1000 ...........NDC 69315-135-10 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a well-closed container as defined in the USP, using a child-resistance closure. Keep this and all Medications out of the reach of children.
Indications & Usage
INDICATIONS AND USAGE Depression - For the relief of symptoms of depression. Endogenous depression is more likely to be alleviated than other depressive states. One to three weeks of treatment may be needed before optimal therapeutic effects are evident. Childhood Enuresis - May be useful as temporary adjunctive therapy in reducing enuresis in children aged 6 years and older, after possible organic causes have been excluded by appropriate tests. In patients having daytime symptoms of frequency and urgency, examination should include voiding cystourethrography and cystoscopy, as necessary. The effectiveness of treatment may decrease with continued drug administration.
Dosage and Administration
DOSAGE AND ADMINISTRATION Depression Lower dosages are recommended for elderly patients and adolescents. Lower dosages are also recommended for outpatients as compared to hospitalized patients who will be under close supervision.Dosage should be initiated at a low level and increased gradually, noting carefully the clinical response and any evidence of intolerance. Following remission, maintenance medication may be required for a longer period of time, at the lowest dose that will maintain remission. Usual Adult Dose Hospitalized Patients - Initially, 100 mg/day in divided doses gradually increased to 200 mg/day as required. If no response after two weeks, increase to 250 to 300 mg/day. Outpatients - Initially, 75 mg/day increased to 150 mg/day. Dosages over 200 mg/day are not recommended. Maintenance, 50 to 150 mg/day. Adolescent and Geriatric Patients - Initially, 30 to 40 mg/day; it is generally not necessary to exceed 100 mg/day. Childhood Enuresis Initially, an oral dose of 25 mg/day should be tried in children aged 6 and older. Medication should be given one hour before bedtime. If a satisfactory response does not occur within one week, increase the dose to 50 mg nightly in children under 12 years; children over 12 may receive up to 75 mg nightly. A daily dose greater than 75 mg does not enhance efficacy and tends to increase side effects. Evidence suggests that in early night bedwetters, the drug is more effective given earlier and in divided amounts, i.e., 25 mg in midafternoon, repeated at bedtime. Consideration should be given to instituting a drug free period following an adequate therapeutic trial with a favorable response, Dosage should be tapered off gradually rather than abruptly discontinued; this may reduce the tendency to relapse. Children who relapse when the drug is discontinued do not always respond to a subsequent course of treatment. A dose of 2.5 mg/kg/day should not be exceeded, ECG changes of unknown significance have been reported in pediatric patients with doses twice this amount. The safety and effectiveness of imipramine hydrochloride tablets, as temporary adjunctive therapy for nocturnal enuresis in children less than 6 years of age has not been established.
