Drug Catalog - Product Detail
IMATINIB MESYLATE 100MG TB 90CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43598-0344-90 | DR.REDDY'S LABORATORIES, INC. | 90 | 100MG | TABLET |
PACKAGE FILES
Generic Name
IMATINIB
Substance Name
IMATINIB MESYLATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA206547
Description
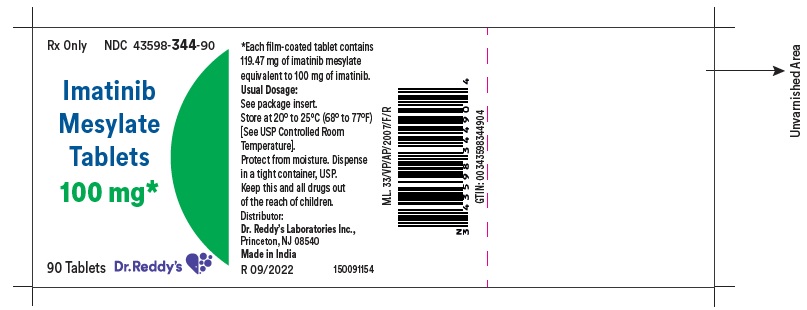
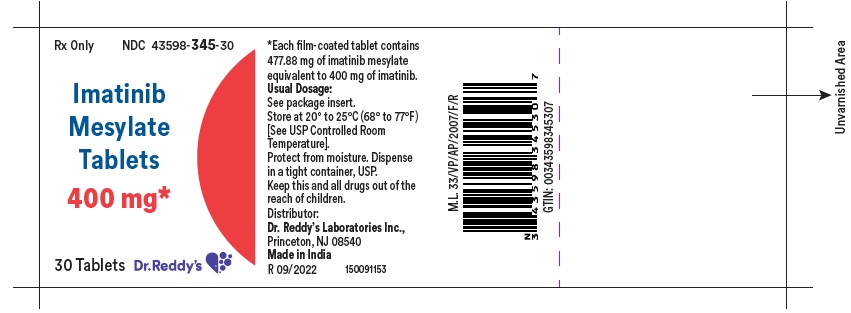
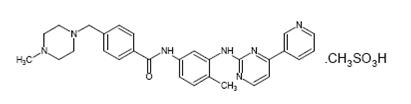
11 DESCRIPTION Imatinib is a small molecule kinase inhibitor. Imatinib mesylate film-coated tablets are supplied as 100 mg and 400 mg tablets for oral administration. Each 100 mg tablet contains 119.47 mg of imatinib mesylate equivalent to 100 mg of imatinib free base. Each 400 mg tablet contains 477.88 mg of imatinib mesylate equivalent to 400 mg of imatinib free base. Imatinib mesylate is designated chemically as 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate and its structural formula is: Imatinib mesylate is an off-white to brownish yellow color powder. Its molecular formula is C 29 H 31 N 7 O • CH 3 SO 3 H and its molecular weight is 589.71 g/mol. It is freely soluble in water. Inactive Ingredients: colloidal silicon dioxide, crospovidone, lactose anhydrous, magnesium stearate (from vegetable source), microcrystalline cellulose, and sodium stearyl fumarate. Tablet coating: hypromellose, macrogol, iron oxide red, iron oxide yellow and titanium dioxide.
How Supplied
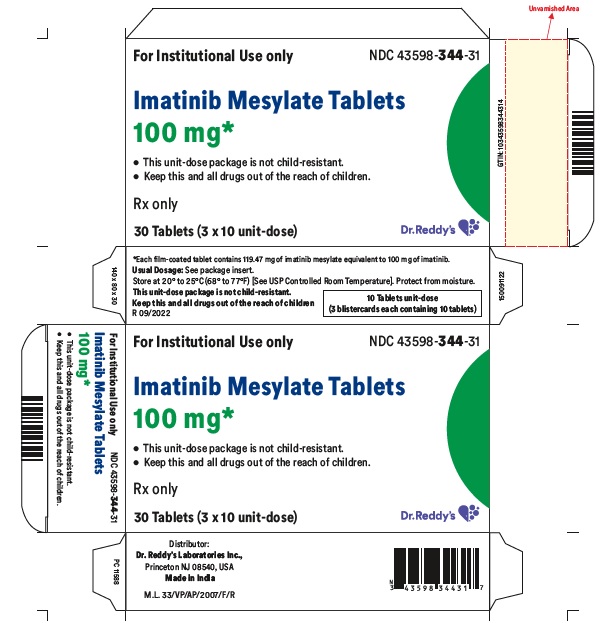
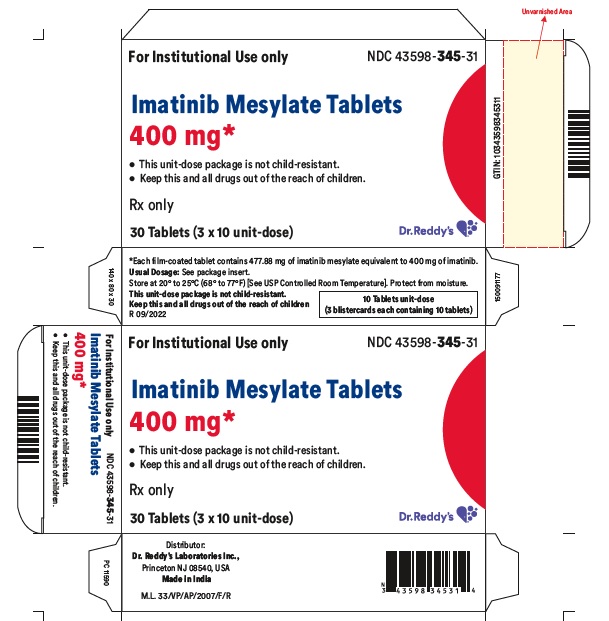
16 HOW SUPPLIED/STORAGE AND HANDLING Each film-coated tablet contains 100 mg or 400 mg of imatinib free base. 100 mg Tablets Yellow to brownish orange, round film coated tablets debossed with “1” on one side and score line on the other side and are supplied in bottles of 30’s, 90’s, 1000’s and unit-dose package of 30 (3x10) Bottles of 30’s NDC 43598-344-30 Bottles of 90’s NDC 43598-344-90 Bottles of 1000’s NDC 43598-344-10 Unit dose package of 30 (3x10) NDC 43598-344-31 400 mg Tablets Yellow to brownish orange, ovaloid film coated tablets debossed with “4” on one side and score line on the other side and are supplied in bottles of 30’s, 90’s, 500’s and unit-dose package of 30 (3x10) . Bottles of 30’s NDC 43598-345-30 Bottles of 90’s NDC 43598-345-90 Bottles of 500’s NDC 43598-345-05 Unit dose package of 30 (3x10) NDC 43598-345-31 Storage and Handling Store at 20° to 25°C (68° to 77°F); [see USP Controlled Room Temperature]. Protect from moisture. Dispense in a tight container, USP. Do not crush imatinib mesylate tablets. Avoid direct contact of crushed tablets with the skin or mucous membranes. If such contact occurs, wash thoroughly as outlined in the references. Avoid exposure to crushed tablets.
Indications & Usage
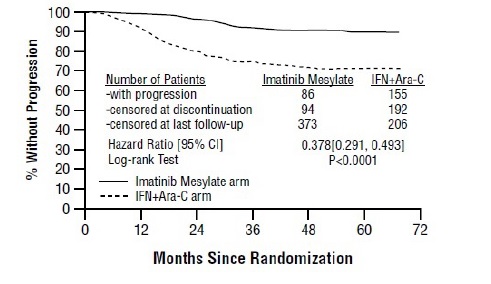
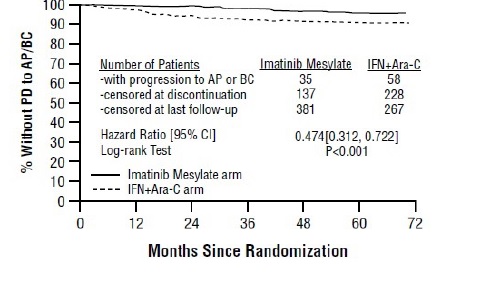
1 INDICATIONS AND USAGE Imatinib mesylate is a kinase inhibitor indicated for the treatment of: Newly diagnosed adult and pediatric patients with Philadelphia chromosome positive chronic myeloid leukemia (Ph+CML) in chronic phase (1.1) Patients with Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) in blast crisis (BC), accelerated phase (AP), or in chronic phase (CP) after failure of interferon-alpha therapy (1.2) Adult patients with relapsed or refractory Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) (1.3) Pediatric patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) in combination with chemotherapy (1.4) Adult patients with myelodysplastic/myeloproliferative diseases (MDS/MPD) associated with platelet-derived growth factor receptor (PDGFR) gene re-arrangements (1.5) Adult patients with aggressive systemic mastocytosis (ASM) without the D816V c-Kit mutation or with c-Kit mutational status unknown (1.6) Adult patients with hypereosinophilic syndrome (HES) and/or chronic eosinophilic leukemia (CEL) who have the FIP1L1-PDGFRα fusion kinase (mutational analysis or fluorescence in situ hybridization [FISH] demonstration of CHIC2 allele deletion) and for patients with HES and/or CEL who are FIP1L1-PDGFRα fusion kinase negative or unknown (1.7) Adult patients with unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans (DFSP) (1.8) Patients with Kit (CD117) positive unresectable and/or metastatic malignant gastrointestinal stromal tumors (GIST) ( 1.9 ) Adjuvant treatment of adult patients following resection of Kit (CD117) positive GIST ( 1.10 ) 1.1 Newly Diagnosed Philadelphia Positive Chronic Myeloid Leukemia (Ph+ CML) Newly diagnosed adult and pediatric patients with Philadelphia chromosome positive chronic myeloid leukemia (Ph+CML) in chronic phase. 1.2 Ph+ CML in Blast Crisis (BC), Accelerated Phase (AP) or Chronic Phase (CP) After Interferon-alpha (IFN) Therapy Patients with Philadelphia chromosome positive chronic myeloid leukemia in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy. 1.3 Adult Patients With Ph+ Acute Lymphoblastic Leukemia (ALL) Adult patients with relapsed or refractory Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL). 1.4 Pediatric Patients With Ph+ Acute Lymphoblastic Leukemia (ALL) Pediatric patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) in combination with chemotherapy. 1.5 Myelodysplastic/Myeloproliferative Diseases (MDS/MPD) Adult patients with myelodysplastic/myeloproliferative diseases associated with platelet-derived growth factor receptor (PDGFR) gene re-arrangements. 1.6 Aggressive Systemic Mastocytosis (ASM) Adult patients with aggressive systemic mastocytosis without the D816V c-Kit mutation or with c-Kit mutational status unknown. 1.7 Hypereosinophilic Syndrome (HES) and/or Chronic Eosinophilic Leukemia (CEL) Adult patients with hypereosinophilic syndrome and/or chronic eosinophilic leukemia who have the FIP1L1-PDGFRα fusion kinase (mutational analysis or fluorescence in situ hybridization [FISH] demonstration of CHIC2 allele deletion) and for patients with HES and/or CEL who are FIP1L1-PDGFRα fusion kinase negative or unknown. 1.8 Dermatofibrosarcoma Protuberans (DFSP) Adult patients with unresectable, recurrent and/or metastatic dermatofibrosarcoma protuberans. 1.9 Kit+ Gastrointestinal Stromal Tumors (GIST) Patients with Kit (CD117) positive unresectable and/or metastatic malignant gastrointestinal stromal tumors. 1.10 Adjuvant Treatment of GIST Adjuvant treatment of adult patients following complete gross resection of Kit (CD117) positive GIST.
Dosage and Administration
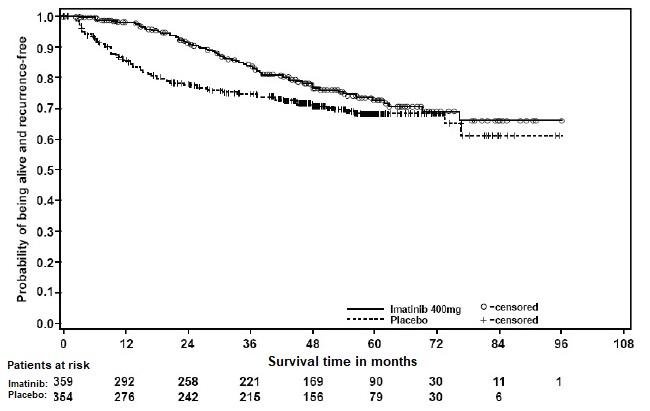
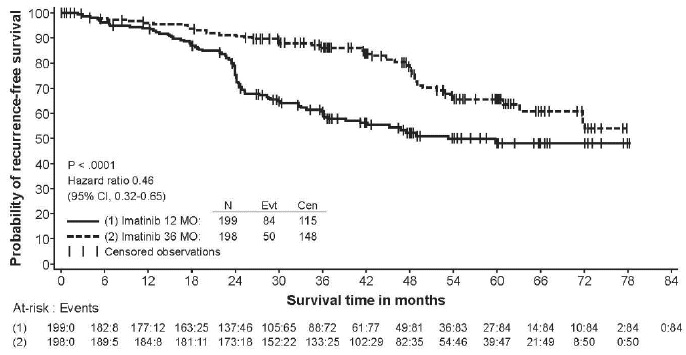
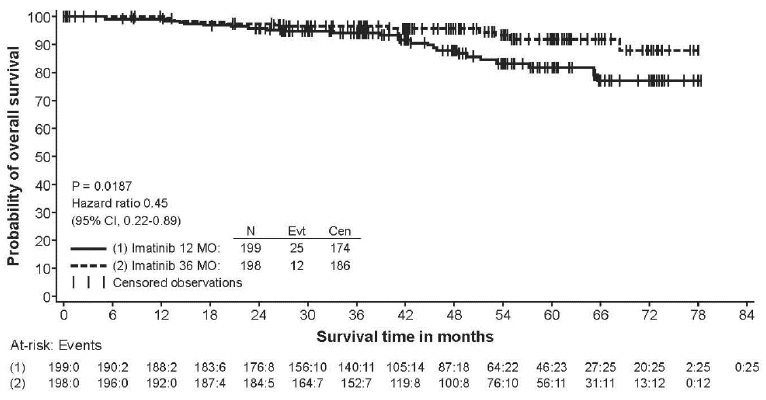
2 DOSAGE AND ADMINISTRATION Adults with Ph+ CML CP (2.2) : 400 mg/day Adults with Ph+ CML AP or BC (2.2) : 600 mg/day Pediatrics with Ph+ CML CP (2.3) : 340 mg/m 2 /day Adults with Ph+ ALL (2.4) : 600 mg/day Pediatrics with Ph+ ALL (2.5) : 340 mg/m 2 /day Adults with MDS/MPD (2.6) : 400 mg/day Adults with ASM (2.7) : 100 mg/day or 400 mg/day Adults with HES/CEL (2.8) : 100 mg/day or 400 mg/day Adults with DFSP (2.9) : 800 mg/day Adults with metastatic and/or unresectable GIST ( 2.10 ): 400 mg/day Adjuvant treatment of adults with GIST ( 2.11 ): 400 mg/day Patients with mild to moderate hepatic impairment (2.12) : 400 mg/day Patients with severe hepatic impairment (2.12) : 300 mg/day All doses of imatinib mesylate tablets should be taken with a meal and a large glass of water. Doses of 400 mg or 600 mg should be administered once daily, whereas a dose of 800 mg should be administered as 400 mg twice a day. Imatinib mesylate tablets can be dissolved in water or apple juice for patients having difficulty swallowing. Daily dosing of 800 mg and above should be accomplished using the 400 mg tablet to reduce exposure to iron. 2.1 Drug Administration The prescribed dose should be administered orally, with a meal and a large glass of water. Doses of 400 mg or 600 mg should be administered once daily, whereas a dose of 800 mg should be administered as 400 mg twice a day. For patients unable to swallow the film-coated tablets, the tablets may be dispersed in a glass of water or apple juice. The required number of tablets should be placed in the appropriate volume of beverage (approximately 50 mL for a 100 mg tablet, and 200 mL for a 400 mg tablet) and stirred with a spoon. The suspension should be administered immediately after complete disintegration of the tablet(s). For daily dosing of 800 mg and above, dosing should be accomplished using the 400 mg tablet to reduce exposure to iron. Treatment may be continued as long as there is no evidence of progressive disease or unacceptable toxicity. 2.2 Adult Patients With Ph+ CML CP, AP, or BC The recommended dose of imatinib mesylate tablets is 400 mg/day for adult patients in chronic phase CML and 600 mg/day for adult patients in accelerated phase or blast crisis. In CML, a dose increase from 400 mg to 600 mg in adult patients with chronic phase disease, or from 600 mg to 800 mg (given as 400 mg twice daily) in adult patients in accelerated phase or blast crisis may be considered in the absence of severe adverse drug reaction and severe non-leukemia related neutropenia or thrombocytopenia in the following circumstances: disease progression (at any time), failure to achieve a satisfactory hematologic response after at least 3 months of treatment, failure to achieve a cytogenetic response after 6 to 12 months of treatment, or loss of a previously achieved hematologic or cytogenetic response. 2.3 Pediatric Patients With Ph+ CML CP The recommended dose of imatinib mesylate tablets for children with newly diagnosed Ph+ CML is 340 mg/m 2 /day (not to exceed 600 mg). Imatinib mesylate treatment can be given as a once daily dose or the daily dose may be split into two–one portion dosed in the morning and one portion in the evening. There is no experience with imatinib mesylate treatment in children under 1 year of age. 2.4 Adult Patients With Ph+ ALL The recommended dose of imatinib mesylate tablets is 600 mg/day for adult patients with relapsed/refractory Ph+ ALL. 2.5 Pediatric Patients With Ph+ ALL The recommended dose of imatinib mesylate tablets to be given in combination with chemotherapy to children with newly diagnosed Ph+ ALL is 340 mg/m 2 /day (not to exceed 600 mg). Imatinib mesylate tablets treatment can be given as a once daily dose. 2.6 Adult Patients With MDS/MPD Determine PDGFRb gene rearrangements status prior to initiating treatment. The recommended dose of imatinib mesylate tablets is 400 mg/day for adult patients with MDS/MPD. 2.7 Adult Patients With ASM Determine D816V c-Kit mutation status prior to initiating treatment. The recommended dose of imatinib mesylate tablets is 400 mg/day for adult patients with ASM without the D816V c-Kit mutation. If c-Kit mutational status is not known or unavailable, treatment with imatinib mesylate tablets 400 mg/day may be considered for patients with ASM not responding satisfactorily to other therapies. For patients with ASM associated with eosinophilia, a clonal hematological disease related to the fusion kinase FIP1L1-PDGFRα, a starting dose of 100 mg/day is recommended. Dose increase from 100 mg to 400 mg for these patients may be considered in the absence of adverse drug reactions if assessments demonstrate an insufficient response to therapy. 2.8 Adult Patients With HES/CEL The recommended dose of imatinib mesylate tablets is 400 mg/day for adult patients with HES/CEL. For HES/CEL patients with demonstrated FIP1L1-PDGFRα fusion kinase, a starting dose of 100 mg/day is recommended. Dose increase from 100 mg to 400 mg for these patients may be considered in the absence of adverse drug reactions if assessments demonstrate an insufficient response to therapy. 2.9 Adult Patients With DFSP The recommended dose of imatinib mesylate tablets is 800 mg/day for adult patients with DFSP. 2.10 Adult Patients With Metastatic and/or Unresectable GIST The recommended dose of imatinib mesylate tablets is 400 mg/day for adult patients with unresectable and/or metastatic, malignant GIST. A dose increase up to 800 mg daily (given as 400 mg twice daily) may be considered, as clinically indicated, in patients showing clear signs or symptoms of disease progression at a lower dose and in the absence of severe adverse drug reactions. 2.11 Adult Patients With Adjuvant GIST The recommended dose of imatinib mesylate tablets is 400 mg/day for the adjuvant treatment of adult patients following complete gross resection of GIST. In clinical trials, one year of imatinib mesylate and three years of imatinib mesylate were studied. In the patient population defined in Study 2, three years of imatinib mesylate is recommended [see Clinical Studies ( 14.8 ) ]. The optimal treatment duration with imatinib mesylate tablets is not known. 2.12 Dose Modification Guidelines Concomitant Strong CYP3A4 inducers: The use of concomitant strong CYP3A4 inducers should be avoided (e.g., dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, rifampacin, phenobarbital). If patients must be coadministered a strong CYP3A4 inducer, based on pharmacokinetic studies, the dosage of imatinib mesylate tablets should be increased by at least 50%, and clinical response should be carefully monitored [see Drug Interactions ( 7.1 )]. Hepatic Impairment: Patients with mild and moderate hepatic impairment do not require a dose adjustment and should be treated per the recommended dose. A 25% decrease in the recommended dose should be used for patients with severe hepatic impairment [see Use in Specific Populations ( 8.6 )]. Renal Impairment: Patients with moderate renal impairment (creatinine clearance [CrCL] = 20 to 39 mL/min) should receive a 50% decrease in the recommended starting dose and future doses can be increased as tolerated. Doses greater than 600 mg are not recommended in patients with mild renal impairment (CrCL=40 to 59 mL/min). For patients with moderate renal impairment doses greater than 400 mg are not recommended. Imatinib should be used with caution in patients with severe renal impairment. A dose of 100 mg/day was tolerated in two patients with severe renal impairment [see Warnings and Precautions ( 5.3 ), Use in Specific Populations ( 8.7 )]. 2.13 Dose Adjustment for Hepatotoxicity and Non-Hematologic Adverse Reactions If elevations in bilirubin greater than 3 times the institutional upper limit of normal (IULN) or in liver transaminases greater than 5 times the IULN occur, imatinib mesylate tablets should be withheld until bilirubin levels have returned to a less than 1.5 times the IULN and transaminase levels to less than 2.5 times the IULN. In adults, treatment with imatinib mesylate tablets may then be continued at a reduced daily dose (i.e., 400 mg to 300 mg, 600 mg to 400 mg or 800 mg to 600 mg). In children, daily doses can be reduced under the same circumstances from 340 mg/m 2 /day to 260 mg/m 2 /day. If a severe non-hematologic adverse reaction develops (such as severe hepatotoxicity or severe fluid retention), imatinib mesylate tablets should be withheld until the event has resolved. Thereafter, treatment can be resumed as appropriate depending on the initial severity of the event. 2.14 Dose Adjustment for Hematologic Adverse Reactions Dose reduction or treatment interruptions for severe neutropenia and thrombocytopenia are recommended as indicated in Table 1. Table 1: Dose Adjustments for Neutropenia and Thrombocytopenia ASM associated with eosinophilia (starting dose 100 mg) ANC 1 less than 1.0 x10 9 /L and/or platelets less than 50 x 10 9 /L 1. 2. Stop imatinib mesylate tablets until ANC greater than or equal to 1.5 x 10 9 /L and platelets greater than or equal to 75x10 9 /L Resume treatment with imatinib mesylate tablets at previous dose (i.e., dose before severe adverse reaction) HES/CEL with FIP1L1-PDGFRα fusion kinase(starting dose 100 mg) ANC less than 1.0 x10 9 /L and/or platelets less than 50 x 10 9 /L 1. 2. Stop imatinib mesylate tablets until ANC greater than or equal to 1.5 x 10 9 /L and platelets greater than or equal to 75x10 9 /L Resume treatment with imatinib mesylate tablets at previous dose (i.e., dose before severe adverse reaction) Chronic Phase CML(starting dose 400 mg) MDS/MPD, ASM and HES/CEL (starting dose 400 mg) GIST (starting dose 400 mg) ANC less than 1.0 x10 9 /L and/or platelets less than 50 x 10 9 /L 1. 2. 3. Stop imatinib mesylate tablets until ANC greater than or equal to 1.5 x 10 9 /L and platelets greater than or equal to 75 x 10 9 /L Resume treatment with imatinib mesylate tablets at the original starting dose of 400 mg If recurrence of ANC less than 1.0 x 10 9 /L and/or platelets less than 50 x 10 9 /L, repeat step 1 and resume imatinib mesylate tablets at a reduced dose of 300 mg Ph+ CML : Accelerated Phase and Blast Crisis (starting dose 600 mg) Ph+ ALL (starting dose 600 mg) ANC less than 0.5 x 10 9 /L and/or platelets less than 10 x 10 9 /L 1. 2. 3. 4. Check if cytopenia is related to leukemia (marrow aspirate or biopsy) If cytopenia is unrelated to leukemia, reduce dose of imatinib mesylate tablets to 400 mg If cytopenia persists 2 weeks, reduce further to 300 mg If cytopenia persists 4 weeks and is still unrelated to leukemia, stop imatinib mesylate tablets until ANC greater than or equal to 1x10 9 /L and platelets greater than or equal to 20 x 10 9 /L and then resume treatment at 300 mg. DFSP (starting dose 800 mg) ANC less than 1.0 x 10 9 /L and/or platelets less than 50 x 10 9 /L 1. 2. 3. Stop imatinib mesylate tablets until ANC greater than or equal to 1.5 x 10 9 /L and platelets greater than or equal to 75 x 10 9 /L Resume treatment with imatinib mesylate tablets at 600 mg In the event of recurrence of ANC less than 1.0 x 10 9 /L and/or platelets less than 50 x 10 9 /L, repeat step 1 and resume imatinib mesylate tablets at reduced dose of 400 mg Pediatric newly diagnosed chronic phase CML (starting dose 340 mg/m 2 ) ANC less than 1.0 x 10 9 /L and/or platelets less than 50 x 10 9 /L 1. 2. 3. Stop imatinib mesylate tablets until ANC greater than or equal to 1.5 x 10 9 /L and platelets greater than or equal to 75 x 10 9 /L Resume treatment with imatinib mesylate tablets at previous dose (i.e., dose before severe adverse reaction) In the event of recurrence of ANC less than 1.0 x10 9 /L and/or platelets less than 50 x 10 9 /L, repeat step 1 and resume imatinib mesylate tablets at reduced dose of 260 mg/m 2 . Abbreviations: ANC, absolute neutrophil count; ASM, aggressive systemic mastocytosis; CEL, chronic eosinophilic leukemia; CML, chronic myeloid leukemia; DFSP, dermatofibrosarcoma protuberans; HES, hypereosinophilic syndrome; MDS/MPD, myelodysplastic/myeloproliferative diseases; PDGFR, platelet-derived growth factor receptor; Ph+ CML, Philadelphia chromosome positive chronic myeloid leukemia; Ph+ ALL, Philadelphia chromosome positive acute lymphoblastic leukemia.
