Drug Catalog - Product Detail
IBUPROFEN 100MG/5ML ORAL SUSP 16 OZ
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00472-2002-16 | ACTAVIS MID ATLANTIC | 473 | 100MG/5ML | NA |
PACKAGE FILES
Generic Name
IBUPROFEN
Substance Name
IBUPROFEN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA074978
Description
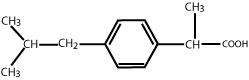
DESCRIPTION The active ingredient in Ibuprofen Oral Suspension, USP is ibuprofen, USP which is a member of the propionic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Ibuprofen, USP is a racemic mixture of [+]S- and [-]R-enantiomers. It is a white to off-white crystalline powder, with a melting point of 74° to 77°C. It is practically insoluble in water (<0.1 mg/mL), but readily soluble in organic solvents such as ethanol and acetone. Ibuprofen, USP has a pKa of 4.43 ± 0.03 and an n-octanol/water partition coefficient of 11.7 at pH 7.4. The chemical name for ibuprofen, USP is (±)-2 - ( p -Isobutylphenyl) propionic acid. The molecular weight of ibuprofen, USP is 206.28. Its molecular formula is C 13 H 18 O 2 and it has the following structural formula: Ibuprofen Oral Suspension, USP is a sucrose-sweetened, orange-colored, berry-flavored suspension containing 100 mg of ibuprofen, USP in 5 mL (20 mg/mL). Inactive ingredients include: acesulfame potassium, citric acid anhydrous, D&C yellow #10, FD&C red #40, glycerin, polysorbate 80, pregelatinized corn starch, purified water, sodium benzoate, strawberry flavor, sucrose and xanthan gum. 1c1b5c78-figure-01
How Supplied
HOW SUPPLIED Ibuprofen Oral Suspension USP, 100 mg/5 mL Orange-colored, berry-flavored suspension - Bottles of 4 fl oz (118 mL) – NDC 0472-2002-94 - Bottles of ONE PINT (473 mL) – NDC 0472-2002-16 Shake well before using. Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP. Distributed by: Actavis Pharma, Inc. Parsippany, NJ 07054 USA Rev. F 7/2024
Indications & Usage
INDICATIONS AND USAGE Carefully consider the potential benefits and risks of Ibuprofen Oral Suspension and other treatment options before deciding to use ibuprofen. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). In Pediatric Patients, Ibuprofen Oral Suspension is indicated: For reduction of fever in patients aged 6 months up to 2 years of age. For relief of mild to moderate pain in patients aged 6 months up to 2 years of age. For relief of signs and symptoms of juvenile arthritis. In Adults, Ibuprofen Oral Suspension is indicated: For treatment of primary dysmenorrhea. For relief of the signs and symptoms of rheumatoid arthritis and osteoarthritis. Since there have been no controlled trials to demonstrate whether there is any beneficial effect or harmful interaction with the use of ibuprofen in conjunction with aspirin, the combination cannot be recommended (see PRECAUTIONS-Drug Interactions ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Carefully consider the potential benefits and risks of ibuprofen oral suspension and other treatment options before deciding to use ibuprofen oral suspension. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ). After observing the response to initial therapy with ibuprofen oral suspension, the dose and frequency should be adjusted to suit an individual patient’s needs. Pediatric Patients Fever Reduction: For reduction of fever in children, 6 months up to 2 years of age, the dosage should be adjusted on the basis of the initial temperature level (see CLINICAL PHARMACOLOGY ). The recommended dose is 5 mg/kg if the baseline temperature is less than 102.5ºF, or 10 mg/kg if the baseline temperature is 102.5ºF or greater. The duration of fever reduction is generally 6 to 8 hours. The recommended maximum daily dose is 40 mg/kg. Analgesia: For relief of mild to moderate pain in children 6 months up to 2 years of age, the recommended dosage is 10 mg/kg, every 6 to 8 hours. The recommended maximum daily dose is 40 mg/kg. Doses should be given so as not to disturb the child's sleep pattern. Juvenile Arthritis: The recommended dose is 30 to 40 mg/kg/day divided into three to four doses (see Individualization of Dosage ). Patients with milder disease may be adequately treated with 20 mg/kg/day. In patients with juvenile arthritis, doses above 50 mg/kg/day are not recommended because they have not been studied and doses exceeding the upper recommended dose of 40 mg/kg/day may increase the risk of causing serious adverse events. The therapeutic response may require from a few days to several weeks to be achieved. Once a clinical effect is obtained, the dosage should be lowered to the smallest dose of ibuprofen oral suspension needed to maintain adequate control of symptoms. Pediatric patients receiving doses above 30 mg/kg/day or if abnormal liver function tests have occurred with previous NSAID treatments should be carefully followed for signs and symptoms of early liver dysfunction. Adults Primary Dysmenorrhea: For the treatment of primary dysmenorrhea, beginning with the earliest onset of such pain, ibuprofen oral suspension should be given in a dose of 400 mg every 4 hours, as necessary, for the relief of pain. Rheumatoid Arthritis and Osteoarthritis: Suggested dosage: 1200 to 3200 mg daily (300 mg q.i.d. or 400 mg, 600 mg or 800 mg t.i.d. or q.i.d.). Individual patients may show a better response to 3200 mg daily, as compared with 2400 mg, although in well-controlled clinical trials patients on 3200 mg did not show a better mean response in terms of efficacy. Therefore, when treating patients with 3200 mg/day, the physician should observe sufficient increased clinical benefits to offset potential increased risk. Individualization of Dosage: The dose of ibuprofen oral suspension should be tailored to each patient, and may be lowered or raised from the suggested doses depending on the severity of symptoms either at time of initiating drug therapy or as the patient responds or fails to respond. One fever study showed that, after the initial dose of ibuprofen oral suspension, subsequent doses may be lowered and still provide adequate fever control. In a situation when low fever would require the ibuprofen oral suspension 5 mg/kg dose in a child with pain, the dose that will effectively treat the predominant symptom should be chosen. In chronic conditions, a therapeutic response to ibuprofen therapy is sometimes seen in a few days to a week, but most often is observed by two weeks. After a satisfactory response has been achieved, the patient's dose should be reviewed and adjusted as required. Patients with rheumatoid arthritis seem to require higher doses than do patients with osteoarthritis. The smallest dose of ibuprofen oral suspension that yields acceptable control should be employed. Ibuprofen oral suspension may be used in combination with gold salts and/or corticosteroids.
