Drug Catalog - Product Detail
IBANDRONATE SODIUM PROPYLENE GLYCOL SOLVATE TB 150MG (160.25MG) 1X3 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 55111-0575-03 | DR.REDDY'S LABORATORIES, INC. | 1 | 150MG | TABLET |
PACKAGE FILES


Generic Name
IBANDRONATE SODIUM
Substance Name
IBANDRONATE SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078997
Description
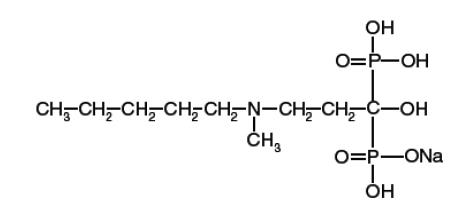
11 DESCRIPTION Ibandronate sodium is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl) amino-1-hydroxypropane-1,1diphosphonic acid, monosodium salt, with the molecular formula C 9 H 22 NO 7 P 2 Na and a molecular weight of 341. Ibandronate sodium is an off-white to white colored powder. It is sparingly soluble in water. Ibandronate sodium has the following structural formula: Ibandronate sodium tablets are available as a white, capsule shaped 150-mg coated tablets for once-monthly oral administration. One 150 mg coated tablet contains 160.33 mg ibandronate sodium, equivalent to 150 mg of ibandronic acid. Ibandronate sodium tablets also contains the following inactive ingredients: colloidal silicon dioxide, crospovidone, croscarmellose sodium, lactose monohydrate, microcrystalline cellulose, povidone, and sodium stearyl fumarate. The tablet coating contains hypromellose, polyethylene glycol 400, and titanium dioxide. Imprinting ink contains: ammonium hydroxide, black iron oxide, propylene glycol and shellac.
How Supplied
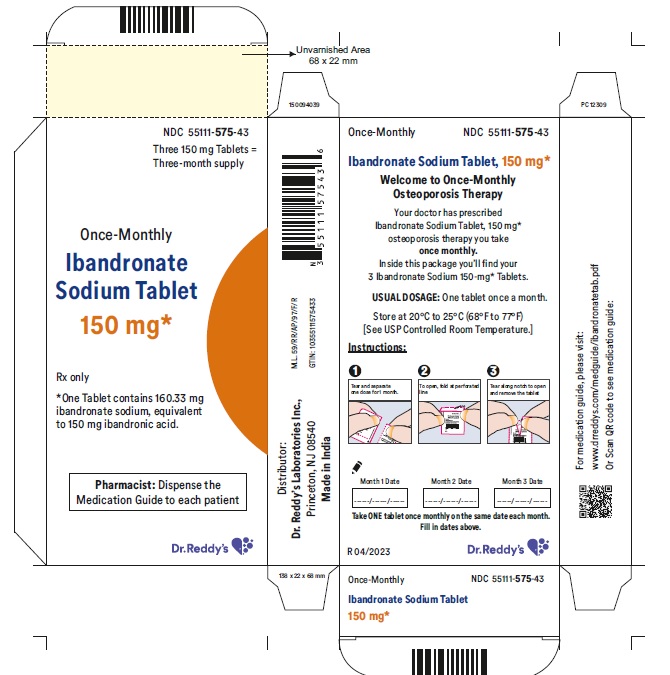
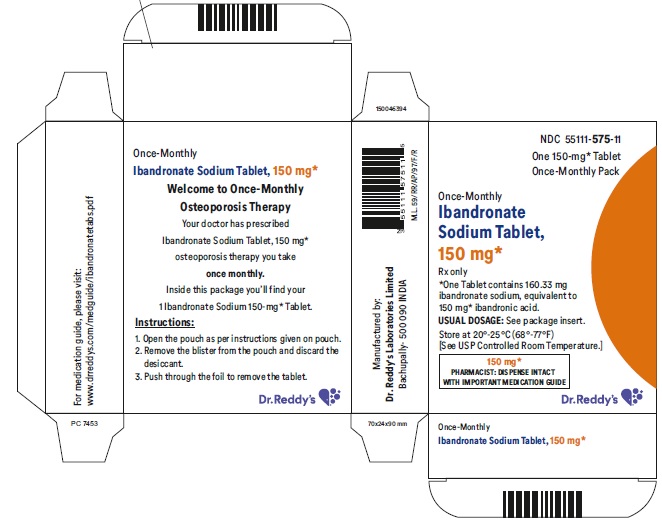
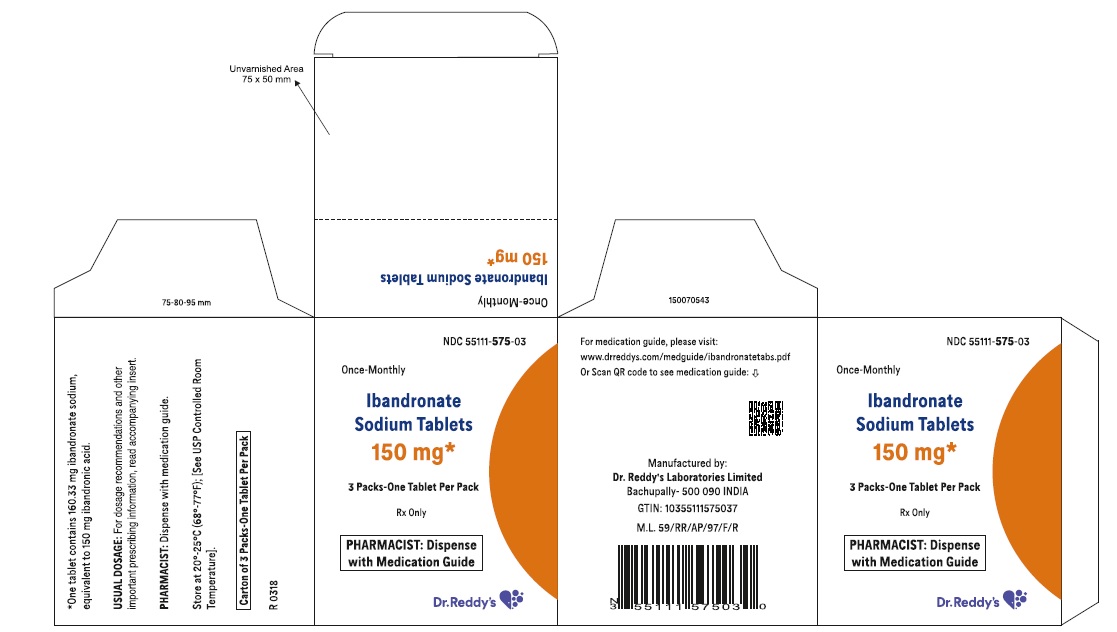
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Ibandronate sodium tablets 150 mg are white, film coated and capsule shaped tablets printed with R575 on one side and plain on other side. Carton of 1 blister pack containing 3 tablets (NDC 55111-575-43). Carton of 3 packs (NDC 55111-575-03), each pack containing 1 tablet (NDC 55111-575-11) 16.2 Storage and Handling Store at 20°C to 25°C (68°F to 77°F); [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Ibandronate sodium tablet is a bisphosphonate indicated for the treatment and prevention of postmenopausal osteoporosis. (1.1) Limitations of Use The optimal duration of use has not been determined. For patients at low-risk for fracture, consider drug discontinuation after 3 to 5 years of use. (1.2). 1.1 Treatment and Prevention of Postmenopausal Osteoporosis Ibandronate sodium tablets are indicated for the treatment and prevention of osteoporosis in postmenopausal women. Ibandronate sodium tablets increases bone mineral density (BMD) and reduces the incidence of vertebral fractures. 1.2 Important Limitations of Use The optimal duration of use has not been determined. The safety and effectiveness of ibandronate sodium tablets for the treatment of osteoporosis are based on clinical data of three years duration. All patients on bisphosphonate therapy should have the need for continued therapy re-evaluated on a periodic basis. Patients at low-risk for fracture should be considered for drug discontinuation after 3 to 5 years of use. Patients who discontinue therapy should have their risk for fracture re-evaluated periodically.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Take one 150 mg tablet once monthly on the same day each month (2.1) Instruct patient to: (2.2) Swallow whole tablet with 6 to 8 oz of plain water only, at least 60 minutes before the first food, beverage, or medication of day. Avoid lying down for at least 60 minutes after taking ibandronate sodium tablets. Do not eat, drink (except for water), or take other medication for 60 minutes after taking ibandronate sodium tablets. Take supplemental calcium and vitamin D if dietary intake inadequate (2.3) 2.1 Dosage Information The dose of ibandronate sodium tablet is one 150 mg tablet taken once monthly on the same date each month. 2.2 Important Administration Instructions Instruct Patients to do the following: Take ibandronate sodium tablets at least 60 minutes before the first food or drink (other than water) of the day or before taking any oral medication or supplementation, including calcium, antacids, or vitamins to maximize absorption and clinical benefit, (see Drug Interactions [7.1] ). Avoid the use of water with supplements including mineral water because they may have a higher concentration of calcium. Swallow ibandronate sodium tablets whole with a full glass of plain water (6 to 8 oz) while standing or sitting in an upright position to reduce the potential for esophageal irritation. Avoid lying down for 60 minutes after taking ibandronate sodium tablets (see Warnings and Precautions [5.1] ). Do not chew or suck the tablet because of a potential for oropharyngeal ulceration. Do not eat, drink anything except plain water, or take other medications for at least 60 minutes after taking ibandronate sodium tablets. 2.3 Recommendations for Calcium and Vitamin D Supplementation Instruct patients to take supplemental calcium and vitamin D if their dietary intake is inadequate. Avoid the use of calcium supplements within 60 minutes of ibandronate sodium tablets administration because co-administration of ibandronate sodium tablets and calcium may interfere with the absorption of ibandronate sodium (see Drug Interactions [7.1] ). 2.4 Administration Instructions for Missed Once-Monthly Doses If the once-monthly dose is missed, instruct patients to do the following: If the next scheduled ibandronate sodium tablets day is more than 7 days away, take one ibandronate sodium tablet, 150 mg in the morning following the date that it is remembered. If the next scheduled ibandronate sodium tablets day is only 1 to 7 days away, wait until the subsequent month’s scheduled ibandronate sodium tablets day to take their tablet. For subsequent monthly doses for both of the above scenarios, instruct patients to return to their original schedule by taking one ibandronate sodium tablet, 150 mg every month on their previous chosen day.
