Drug Catalog - Product Detail
HYDROXYZINE HCL TB 50MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 23155-0502-10 | AVET PHARMACEUTICALS | 1000 | 50MG | TABLET |
PACKAGE FILES


Generic Name
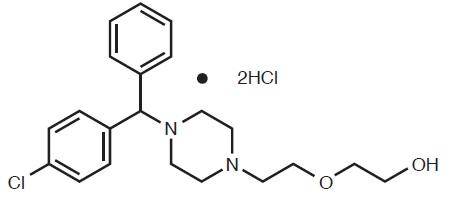
HYDROXYZINE HYDROCHLORIDE
Substance Name
HYDROXYZINE DIHYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204279
Description
DESCRIPTION Hydroxyzine hydrochloride, USP has the chemical name of (±)-2-[2-[4-( p -Chloro-α-phenylbenzyl)-1-piperazinyl]ethoxy]ethanol dihydrochloride. Molecular Formula: C 21 H 27 ClN 2 O 2 · 2HCl Molecular Weight: 447.83 Hydroxyzine hydrochloride, USP occurs as a white, odorless powder which is very soluble in water. Each tablet for oral administration contains 10 mg, 25 mg, or 50 mg hydroxyzine hydrochloride, USP. Inactive ingredients include anhydrous lactose, colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate and titanium dioxide. stucture
How Supplied
HOW SUPPLIED Hydroxyzine Hydrochloride Tablets, USP are available as follows: 10 mg tablets: round, film coated white tablets. Debossed H on one side and 500 on the reverse side. They are available as follows: Bottles of 100: NDC 23155-500-01 Bottles of 500: NDC 23155-500-05 Bottles of 1000: NDC 23155-500-10 25 mg tablets: round, film coated white tablets. Debossed H over 501 on one side and plain on the reverse side. They are available as follows: Bottles of 100: NDC 23155-501-01 Bottles of 500: NDC 23155-501-05 Bottles of 1000: NDC 23155-501-10 50 mg tablets: round, film coated white tablets. Debossed H over 502 on one side and plain on the reverse side. They are available as follows: Bottles of 100: NDC 23155-502-01 Bottles of 500: NDC 23155-502-05 Bottles of 1000: NDC 23155-502-10 Dispense in a tight container as defined in the USP, with a child-resistant closure (as required). Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. Distributed by: Avet Pharmaceuticals Inc. East Brunswick, NJ 08816 1.866.901.DRUG (3784) 51U000000193US08 Rev. 09/2021 logo
Indications & Usage
INDICATIONS AND USAGE For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested. Useful in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses and in histamine-mediated pruritus. As a sedative when used as a premedication and following general anesthesia, hydroxyzine may potentiate meperidine and barbiturates, so their use in pre-anesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. Hydroxyzine is not known to interfere with the action of digitalis in any way and it may be used concurrently with this agent. The effectiveness of hydroxyzine as an antianxiety agent for long term use, that is more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient.
Dosage and Administration
DOSAGE AND ADMINISTRATION For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested: adults, 50 to 100 mg q.i.d.; children under 6 years, 50 mg daily in divided doses; children over 6 years, 50 to 100 mg daily in divided doses. For use in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses and in histamine-mediated pruritus: adults, 25 mg t.i.d. or q.i.d.; children under 6 years, 50 mg daily in divided doses; children over 6 years, 50 to 100 mg daily in divided doses. As a sedative when used as a premedication and following general anesthesia: 50 to 100 mg for adults and 0.6 mg/kg of body weight in children. When treatment is initiated by the intramuscular route of administration, subsequent doses may be administered orally. As with all potent medication, the dosage should be adjusted according to the patient's response to therapy.
