Drug Catalog - Product Detail
HYDROCORTISONE ENEMA 100 MG/60ML 60 ML X 7
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62559-0138-07 | ANI PHARMACEUTICALS | 60 | 100MG/60ML | ENEMA |
PACKAGE FILES

Generic Name
HYDROCORTISONE
Substance Name
HYDROCORTISONE
Product Type
HUMAN PRESCRIPTION DRUG
Route
RECTAL
Application Number
NDA016199
Description
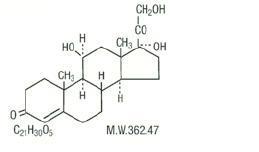
DESCRIPTION Hydrocortisone is a white to practically white, odorless, crystalline powder, very slightly soluble in water. It has the chemical name Pregn-4-ene-3,20-dione,11,17,21-trihydroxy-,(11β)- and the following structural formula: Hydrocortisone Rectal Suspension, USP is a convenient disposable single-dose hydrocortisone enema designed for ease of self-administration. Each disposable unit (60 mL) contains: Hydrocortisone, 100 mg in an aqueous solution containing carbomer 934P, polysorbate 80, purified water, sodium hydroxide and methylparaben, 0.18% as a preservative. Chemical Structure
How Supplied
HOW SUPPLIED Hydrocortisone Rectal Suspension, USP (Retention)100 mg is supplied as disposable single-dose bottles with lubricated rectal applicator tips, in boxes of seven x 60 mL (NDC 62559-138-07) and boxes of one x 60 mL (NDC 62559-138-11). Store at controlled room temperature 20° - 25°C (68° - 77°F). [See USP Controlled Room Temperature.]
Indications & Usage
INDICATIONS AND USAGE Hydrocortisone Rectal Suspension, USP is indicated as adjunctive therapy in the treatment of ulcerative colitis, especially distal forms, including ulcerative proctitis, ulcerative proctosigmoiditis, and left-sided ulcerative colitis. It has proved useful also in some cases involving the transverse and ascending colons.
Dosage and Administration
DOSAGE AND ADMINISTRATION The use of Hydrocortisone Rectal Suspension, USP hydrocortisone retention enema is predicated upon the concomitant use of modern supportive measures such as rational dietary control, sedatives, antidiarrheal agents, antibacterial therapy, blood replacement if necessary, etc. The usual course of therapy is one Hydrocortisone Rectal Suspension, USP nightly for 21 days, or until the patient comes into remission both clinically and proctologically. Clinical symptoms usually subside promptly within 3 to 5 days. Improvement in the appearance of the mucosa, as seen by sigmoidoscopic examination, may lag somewhat behind clinical improvement. Difficult cases may require as long as 2 or 3 months of Hydrocortisone Rectal Suspension, USP treatment. Where the course of therapy extends beyond 21 days, Hydrocortisone Rectal Suspension, USP should be discontinued gradually by reducing administration to every other night for 2 or 3 weeks. If clinical or proctologic improvement fails to occur within 2 or 3 weeks after starting Hydrocortisone Rectal Suspension, USP, discontinue its use. Symptomatic improvement, evidenced by decreased diarrhea and bleeding; weight gain; improved appetite; lessened fever; and decrease in leukocytosis, may be misleading and should not be used as the sole criterion in judging efficacy. Sigmoidoscopic examination and X-ray visualization are essential for adequate monitoring of ulcerative colitis. Biopsy is useful for differential diagnosis. Patient instructions for administering Hydrocortisone Rectal Suspension, USP are enclosed in each box. It is recommended that the patient lie on their left side during administration and for 30 minutes thereafter, so that the fluid will distribute throughout the left colon. Every effort should be made to retain the enema for at least an hour and preferably, all night. This may be facilitated by prior sedation and/or antidiarrheal medication, especially early in therapy when the urge to evacuate is great.
