Drug Catalog - Product Detail
HYDROCORTISONE BUTYRATE CRM 0.1% 45GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68682-0384-45 | OCEANSIDE PHARMACEUTICALS | 45 | 0.1% | CREAM |
PACKAGE FILES

Generic Name
HYDROCORTISONE BUTYRATE
Substance Name
HYDROCORTISONE BUTYRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
NDA020769
Description
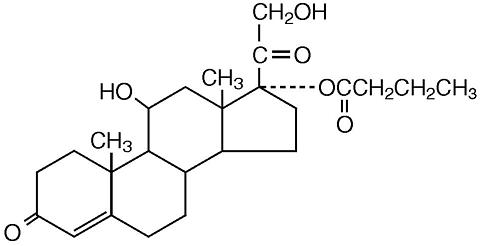
11 DESCRIPTION Hydrocortisone Butyrate Cream (lipid), 0.1% contains hydrocortisone butyrate, a non-fluorinated hydrocortisone ester, for topical use. The chemical name of hydrocortisone butyrate is 11ß,17,21-Trihydroxypregn-4-ene-3,20-dione 17-butyrate. It has the following structural formula: Hydrocortisone butyrate is a white to off-white powder with a molecular weight of 432.56, and a molecular formula of C 25 H 36 O 6 . It is practically insoluble in water, slightly soluble in ether, soluble in methanol, in alcohol, and in acetone, and freely soluble in chloroform. Each gram of Hydrocortisone Butyrate Cream (lipid), 0.1% contains 1 mg of hydrocortisone butyrate in a white to off-white hydrophilic cream base consisting of anhydrous citric acid, butylparaben, ceteth-20, cetostearyl alcohol, mineral oil, propylparaben, purified water, sodium citrate, and white petrolatum. hydrocortisone butyrate structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Hydrocortisone Butyrate Cream (lipid), 0.1% is white to off-white in color, and supplied in tubes of 45 g (NDC 68682-384-45) and 60 g (NDC 68682-384-60). Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from freezing.
Indications & Usage
1 INDICATIONS AND USAGE Hydrocortisone Butyrate Cream (lipid), 0.1% is indicated for: • Relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in adults. • The topical treatment of mild to moderate atopic dermatitis in pediatric patients 3 months to 18 years of age. Hydrocortisone Butyrate Cream (lipid), 0.1% is a corticosteroid indicated for: • Relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in adults. ( 1 ) • The treatment of mild to moderate atopic dermatitis in patients 3 months to 18 years of age. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION For corticosteroid-responsive dermatoses in adults, apply a thin layer to the affected skin areas 2 or 3 times daily, depending on the severity of the condition, and rub in gently. For atopic dermatitis in patients 3 months to 18 years of age, apply a thin layer to the affected skin areas 2 times daily and rub in gently. Do not apply Hydrocortisone Butyrate Cream (lipid), 0.1% in the diaper area unless directed by a physician. Discontinue therapy when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary. Before prescribing for more than 2 weeks, any additional benefits of extending treatment to 4 weeks should be weighed against the risk of HPA-axis suppression and local adverse events. The safety and efficacy of Hydrocortisone Butyrate Cream (lipid), 0.1% has not been established beyond 4 weeks of use [see Warnings and Precautions (5.1) ] . Do not use Hydrocortisone Butyrate Cream (lipid), 0.1% with occlusive dressings unless directed by a physician. Avoid use in the diaper area, as diapers or plastic pants may constitute occlusive dressings. Hydrocortisone Butyrate Cream (lipid), 0.1% is not for oral, ophthalmic, or intravaginal use. • Apply a thin layer to the affected skin areas 2 or 3 times daily for corticosteroid-responsive dermatoses in adults. ( 2 ) • Apply a thin layer to the affected skin areas 2 times daily for atopic dermatitis in patients 3 months of age and older. ( 2 ) • Rub in gently. ( 2 ) • Discontinue Hydrocortisone Butyrate Cream (lipid), 0.1% when control is achieved. ( 2 ) • Reassess diagnosis if no improvement is seen within 2 weeks. Before prescribing for more than 2 weeks, any additional benefits of extending treatment to 4 weeks should be weighed against the risk of hypothalamic-pituitary-adrenal (HPA) axis suppression and local adverse events. Safety and efficacy of Hydrocortisone Butyrate Cream (lipid), 0.1% has not been established beyond 4 weeks of use. ( 2 ) • Avoid use under occlusion or in the diaper area. ( 2 ) • Hydrocortisone Butyrate Cream (lipid), 0.1% is not for oral, ophthalmic, or intravaginal use. ( 2 )
