Drug Catalog - Product Detail
HYDROCORTISONE ACET (PROCTO-MED HC CREAM) CRM 0.025 1OZ
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69315-0302-30 | LEADING PHARMA | 30 | 2.5% | CREAM |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
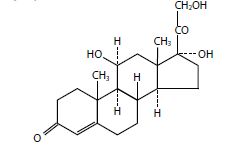
Rx Only DESCRIPTION The topical corticosteroids constitute a class of primarily syn thetic steroids used as anti-inflammatory and anti- pruritic agents. Hydrocortisone cream is a member of this class. Hydrocortisone cream contains the synthetic steroid hydrocortisone (Pregn-4-ene-3,20-dione, 11, 17, 21-tri hydroxy-, (11ß)-) which has a molecular formula of C 21 H 30 O 5 , a molecular weight of 362.46 and CAS Registry Number 50-23-7. Each gram of the 2.5% cream contains 25 mg of hydrocortisone USP in a cream base of cetyl alcohol, citric acid, glyceryl stearate, isopropyl myristate, methylparaben, polyoxyl 40 stearate, polysorbate 60, propylene glycol, propylparaben, purified water, sodium citrate, sorbic acid, sorbitan monostearate, stearyl alcohol, white wax and citric acid solution and sodium citrate solution to adjust pH. furosemide structure
How Supplied
HOW SUPPLIED Hydrocortisone Cream USP, 2.5% is available as follows: 30 g (1.1 oz) tube (NDC 69315-302-30) Store at controlled room temperature 15°-30°C (59°-86°F). Do not freeze. Manufactured by: G &W Laboratories, Inc. 111 Coolidge Street South Plainfield, NJ 07080 USA Distributed by: Leading Pharma, LLC Fairfield, NJ 07004 Revised – January 2016 365I600-0613 GW 7067
Indications & Usage
INDICATIONS AND USAGE Topical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of cortico steroid-responsive dermatoses.
Dosage and Administration
DOSAGE AND ADMINISTRATION Topical corticosteroids are generally applied to the affected area as a thin film from 2 to 4 times daily depending on the severity of the condition. Occlusive dressings may be used for the management of psoriasis or recalcitrant conditions. If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
