Drug Catalog - Product Detail
GUANFACINE HCL TB 2MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0713-10 | AMNEAL PHARMACEUTICALS | 100 | 2MG | TABLET |
PACKAGE FILES


Generic Name
GUANFACINE
Substance Name
GUANFACINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075109
Description
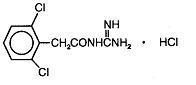
DESCRIPTION Guanfacine hydrochloride, USP is a centrally acting antihypertensive with α 2 -adrenoceptor agonist properties in tablet form for oral administration. The chemical name of guanfacine hydrochloride, USP is N-amidino-2-(2,6-dichlorophenyl) acetamide hydrochloride and its molecular weight is 282.56. Its structural formula is: Guanfacine hydrochloride, USP is a white to off-white powder; sparingly soluble in water and alcohol and slightly soluble in acetone. Each tablet, for oral administration, contains guanfacine hydrochloride, USP equivalent to 1 mg or 2 mg guanfacine. In addition, each tablet contains the following inactive ingredients: microcrystalline cellulose, pregelatinized starch, and stearic acid. 042bf3b6-figure-01
How Supplied
HOW SUPPLIED Guanfacine tablets, USP are available in 2 tablet strengths of guanfacine (as the hydrochloride salt) as follows: 1 mg: white, oval, flat-faced, beveled-edge tablet with “AN” on one side and “711” on the other side. They are available as follows: Bottles of 30: NDC 65162-711-03 Bottles of 100: NDC 65162-711-10 2 mg: white, oval, flat-faced, beveled-edge tablet with “AN” on one side and “713” on the other side. They are available as follows: Bottles of 30: NDC 65162-713-03 Bottles of 100: NDC 65162-713-10 Bottles of 500: NDC 65162-713-50 Store at 20º to 25 ºC (68 ºF to 77 ºF) [see USP Controlled Room Temperature]. Dispense in tight, light-resistant container. Packaged with child-resistant closure. Distributed by: Amneal Pharmaceuticals Bridgewater, NJ 08807 Rev. 11-2015-00
Indications & Usage
INDICATIONS & USAGE Guanfacine tablets, USP are indicated in the management of hypertension. Guanfacine may be given alone or in combination with other antihypertensive agents, especially thiazide-type diuretics.
Dosage and Administration
DOSAGE & ADMINISTRATION The recommended initial dose of guanfacine tablets, USP when given alone or in combination with another antihypertensive drug is 1 mg daily given at bedtime to minimize somnolence. If after 3 to 4 weeks of therapy 1 mg does not give a satisfactory result, a dose of 2 mg may be given, although most of the effect of guanfacine is seen at 1 mg (see CLINICAL PHARMACOLOGY ). Higher daily doses have been used, but adverse reactions increase significantly with doses above 3 mg/day. The frequency of rebound hypertension is low, but it can occur. When rebound occurs, it does so after 2 to 4 days, which is delayed compared with clonidine hydrochloride. This is consistent with the longer half-life of guanfacine. In most cases, after abrupt withdrawal of guanfacine, blood pressure returns to pretreatment levels slowly (within 2 to 4 days) without ill effects.
