Drug Catalog - Product Detail
GRANISETRON HCL FOR INJECTION INJECT. 1MG/ML 10X1ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 17478-0547-01 | AKORN | NA | NA | NA |
PACKAGE FILES




Generic Name
Substance Name
Product Type
Route
Application Number
Description
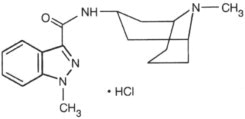
11 DESCRIPTION Granisetron Hydrochloride Injection, USP is a serotonin-3 (5-HT 3 ) receptor antagonist. Chemically it is endo -N-(9-methyl-9-azabicyclo [3.3.1] non-3-yl)-1-methyl-1H-indazole-3-carboxamide hydrochloride. Its chemical structure is: Granisetron hydrochloride C 18 H 24 N 4 O•HCl M.W. 348.9 (312.4 free base) Granisetron hydrochloride is a white to off-white solid that is readily soluble in water and normal saline at 20°C. Granisetron hydrochloride injection, USP is a clear, colorless, sterile, nonpyrogenic, aqueous solution for intravenous administration. Granisetron hydrochloride injection, USP 1 mg/mL is available in 1 mL Single-dose and 4 mL Multiple-dose vials. Granisetron hydrochloride injection, USP 0.1 mg/mL is available in a 1 mL single-dose vial. 1 mg/mL: Each 1 mL contains: Active: Granisetron hydrochloride 1.12 mg, equivalent to granisetron, 1 mg; Inactives: Sodium Chloride, 9 mg; Citric Acid, 2 mg; Sodium Citrate, Dihydrate, Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (approximately 4.0 to 6.0), and Water for Injection q.s. to 1 mL; Preservative: Benzyl Alcohol, 10 mg. 0.1 mg/mL (preservative-free): Each mL contains: Active: Granisetron hydrochloride 0.112 mg, equivalent to granisetron, 0.1 mg; Inactives: Sodium Chloride, 9 mg; Citric Acid, 2 mg: Sodium Citrate, Dihydrate, Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH (approximately 4.0 to 6.0), and Water for Injection q.s. to 1 mL. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Granisetron Hydrochloride Injection, USP, 1 mg/mL (free base), is supplied in 1 mL Single-dose vials and 4 mL Multi-dose vials. CONTAINS BENZYL ALCOHOL. NDC 17478-546-02 (package of 1 Single-dose Vial) NDC 17478-546-05 (package of 1 Multi-dose Vial) Granisetron Hydrochloride Injection, USP 0.1 mg/mL (free base), is supplied in 1 mL Single-dose Vials. CONTAINS NO PRESERVATIVE. NDC 17478-547-01 (package of 10 Single-dose Vial) Storage: Store single-dose vials and multiple-dose vials at 20º to 25°C (68º to 77°F) [see USP Controlled Room Temperature]. Protect from light. Do not freeze. Retain in carton until time of use. Once the multiple-dose vial is penetrated, its contents should be used within 30 days.
Indications & Usage
1 INDICATIONS AND USAGE Granisetron Hydrochloride Injection, USP is a serotonin-3 (5-HT 3 ) receptor antagonist indicated for: The prevention of nausea and/or vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin. Granisetron Hydrochloride Injection, USP is a serotonin-3 (5-HT 3 ) receptor antagonist indicated for: The prevention of nausea and/or vomiting associated with initial and repeat courses of emetogenic cancer therapy, including high-dose cisplatin. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Prevention of chemotherapy-induced nausea and vomiting ( 2.1 ): Recommended dosage is 10 mcg/kg intravenously within 30 minutes before initiation of chemotherapy Pediatric patients (2 to 16 years): Recommended dosage is 10 mcg/kg 2.1 Prevention of Chemotherapy-Induced Nausea and Vomiting Adult Patients The recommended dosage for granisetron hydrochloride injection is 10 mcg/kg administered intravenously within 30 minutes before initiation of chemotherapy, and only on the day(s) chemotherapy is given. Infusion Preparation Granisetron hydrochloride injection may be administered intravenously either undiluted over 30 seconds, or diluted with 0.9% Sodium Chloride or 5% Dextrose and infused over 5 minutes. Stability Intravenous infusion of granisetron hydrochloride injection should be prepared at the time of administration. However, granisetron hydrochloride injection has been shown to be stable for at least 24 hours when diluted in 0.9% Sodium Chloride or 5% Dextrose and stored at room temperature under normal lighting conditions. As a general precaution, granisetron hydrochloride injection should not be mixed in solution with other drugs. Parenteral drug products should be inspected visually for particulate matter and discoloration before administration whenever solution and container permit. Pediatric Patients The recommended dose in pediatric patients 2 to 16 years of age is 10 mcg/kg [see Clinical Studies ( 14 )] . Pediatric patients under 2 years of age have not been studied.
