Drug Catalog - Product Detail
FOLIC ACID USP TB 1MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69315-0127-10 | LEADING PHARMA | 1000 | 1MG | TABLET |
PACKAGE FILES


Generic Name
FOLIC ACID
Substance Name
FOLIC ACID
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040796
Description
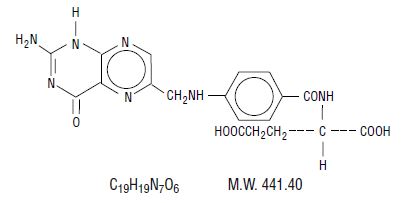
DESCRIPTION Folic acid, N-p-[[(2-amino-4-hydroxy-6-pteridinyl) methyl]amino] benzoyl]- L-glutamic acid, is a B complex vitamin containing a pteridine moiety linked by a methylene bridge to para- aminobenzoic acid, which is joined by a peptide linkage to glutamic acid. Conjugates of folic acid are present in a wide variety of foods, particularly liver, kidneys, yeast and leafy green vegetables. Commercially available folic acid is prepared synthetically. Folic acid occurs as a yellow or yellowish-orange crystalline powder and is very slightly soluble in water and insoluble in alcohol. Folic acid is readily soluble in dilute solutions of alkali hydroxides and carbonates, and solutions of the drug may be prepared with the aid of sodium hydroxide or sodium carbonate, thereby forming the soluble sodium salt of folic acid (sodium folate). Aqueous solutions of folic acid are heat sensitive and rapidly decompose in the presence of light and/or riboflavin; solutions should be stored in a cool place protected from light. The structural formula of folic acid is as follows: Each tablet, for oral administration, contains 1 mg folic acid. Folic acid tablets, USP 1 mg contain the following inactive ingredients: corn starch, microcrystalline cellulose, sodium starch glycolate and stearic acid. Chemical structure
How Supplied
HOW SUPPLIED Folic acid tablets, USP 1 mg are yellow to orange color, round, bisected compressed tablets, debossed “EP” above bisect and “127” below the bisect on one side and plain on the other side in bottles of 100 (NDC 69315-127-01) and 1000 (NDC 69315-127-10). Dispense in a well-closed container as defined in the USP, using a child-resistant closure. Store at 20°-25° C (68°-77° F). [See USP controlled room temperature.] Manufactured by: Leading Pharma, LLC Fairfield, NJ 07004 Rev. 02 04/17
Indications & Usage
INDICATIONS AND USAGE Folic acid is effective in the treatment of megaloblastic anemias due to a deficiency of folic acid (as may be seen in tropical or nontropical sprue) and in anemias of nutritional origin, pregnancy, infancy, or childhood.
Dosage and Administration
DOSAGE AND ADMINISTRATION Oral administration is preferred. Although most patients with malabsorption cannot absorb food folates, they are able to absorb folic acid given orally. Parenteral administration is not advocated but may be necessary in some individuals (e.g., patients receiving parenteral or enteral alimentation). Doses greater than 0.1 mg should not be used unless anemia due to vitamin B 12 deficiency has been ruled out or is being adequately treated with a cobalamin. Daily doses greater than 1 mg do not enhance the hematologic effect, and most of the excess is excreted unchanged in the urine. The usual therapeutic dosage in adults and children (regardless of age) is up to 1 mg daily. Resistant cases may require larger doses. When clinical symptoms have subsided and the blood picture has become normal, a daily maintenance level should be used, i.e., 0.1 mg for infants and up to 0.3 mg for children under 4 years of age, 0.4 mg for adults and children 4 or more years of age, and 0.8 mg for pregnant and lactating women, but never less than 0.1 mg/day. Patients should be kept under close supervision and adjustment of the maintenance level made if relapse appears imminent. In the presence of alcoholism, hemolytic anemia, anticonvulsant therapy, or chronic infection, the maintenance level may need to be increased.
