Drug Catalog - Product Detail
FLUTICASONE PROPIONATE/SALMETEROL INH 250/50MCG SOL 60
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 66993-0585-97 | PRASCO LABORATORIES | 60 | 250-50MCG/ACT | AEROSOL |
PACKAGE FILES










Generic Name
FLUTICASONE PROPIONATE AND SALMETEROL
Substance Name
FLUTICASONE PROPIONATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
RESPIRATORY (INHALATION)
Application Number
NDA021077
Description
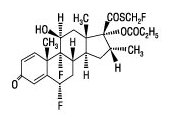
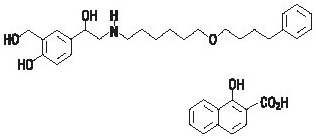
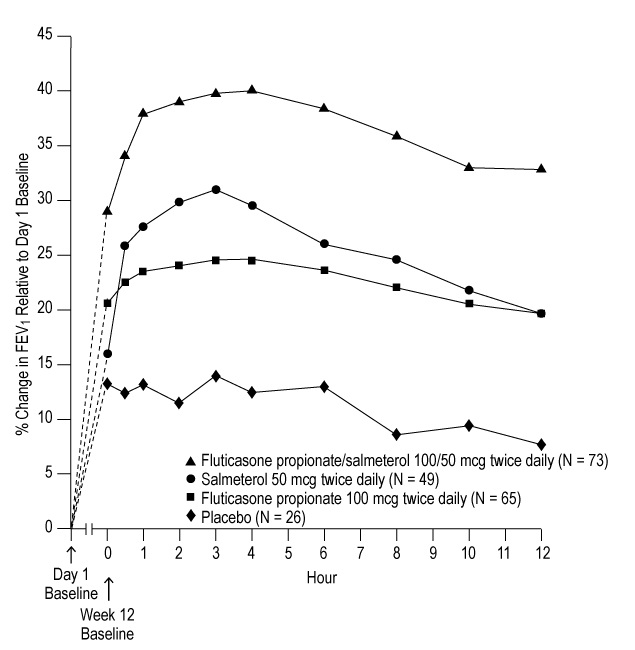
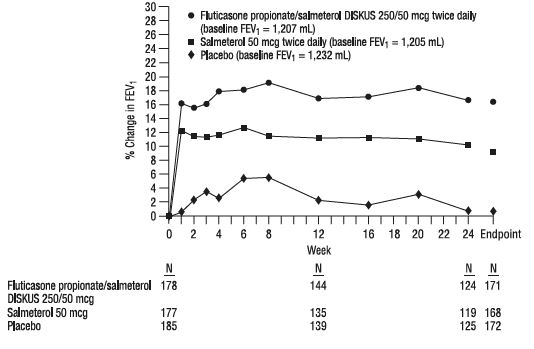
11 DESCRIPTION Fluticasone Propionate/Salmeterol DISKUS inhalation powder 100/50 mcg, Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg, and Fluticasone Propionate/Salmeterol DISKUS inhalation powder 500/50 mcg are combinations of fluticasone propionate and salmeterol xinafoate. One active component of Fluticasone Propionate/Salmeterol DISKUS is fluticasone propionate, a corticosteroid having the chemical name S- (fluoromethyl) 6α,9-difluoro-11β,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate, 17-propionate and the following chemical structure: Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C 25 H 31 F 3 O 5 S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol. The other active component of Fluticasone Propionate/Salmeterol DISKUS is salmeterol xinafoate, a beta 2 -adrenergic bronchodilator. Salmeterol xinafoate is the racemic form of the 1-hydroxy-2-naphthoic acid salt of salmeterol. It has the chemical name 4-hydroxy-α 1 -[[[6-(4-phenylbutoxy)hexyl]amino]methyl]-1,3-benzenedimethanol, 1-hydroxy-2-naphthalenecarboxylate and the following chemical structure: Salmeterol xinafoate is a white powder with a molecular weight of 603.8, and the empirical formula is C 25 H 37 NO 4 •C 11 H 8 O 3 . It is freely soluble in methanol; slightly soluble in ethanol, chloroform, and isopropanol; and sparingly soluble in water. Fluticasone Propionate/Salmeterol DISKUS is a purple plastic inhaler containing a foil blister strip. Each blister on the strip contains a white powder mix of micronized fluticasone propionate (100, 250, or 500 mcg) and micronized salmeterol xinafoate salt (72.5 mcg, equivalent to 50 mcg of salmeterol base) in 12.5 mg of formulation containing lactose monohydrate (which contains milk proteins). After the inhaler is activated, the powder is dispersed into the airstream created by the patient inhaling through the mouthpiece. Under standardized in vitro test conditions, Fluticasone Propionate/Salmeterol DISKUS delivers 93, 233, and 465 mcg of fluticasone propionate and 45 mcg of salmeterol base per blister from Fluticasone Propionate/Salmeterol DISKUS inhalation powder 100/50 mcg, Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg, and Fluticasone Propionate/Salmeterol DISKUS inhalation powder 500/50 mcg, respectively, when tested at a flow rate of 60 L/min for 2 seconds. In adult subjects with obstructive lung disease and severely compromised lung function (mean FEV 1 20% to 30% of predicted), mean peak inspiratory flow (PIF) through the DISKUS inhaler was 82.4 L/min (range: 46.1 to 115.3 L/min). Inhalation profiles for adolescent (N = 13, aged 12 to 17 years) and adult (N = 17, aged 18 to 50 years) subjects with asthma inhaling maximally through the DISKUS inhaler show mean PIF of 122.2 L/min (range: 81.6 to 152.1 L/min). Inhalation profiles for pediatric subjects with asthma inhaling maximally through the DISKUS inhaler show a mean PIF of 75.5 L/min (range: 49.0 to 104.8 L/min) for the 4-year-old subject set (N = 20) and 107.3 L/min (range: 82.8 to 125.6 L/min) for the 8-year-old subject set (N = 20). The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile. Fluticasone propionate chemical structure Salmeterol xinafoate chemical structure
How Supplied
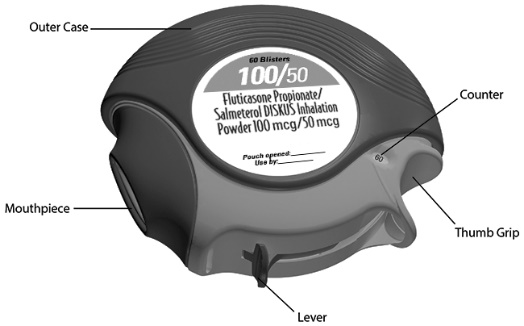
16 HOW SUPPLIED/STORAGE AND HANDLING Fluticasone Propionate/Salmeterol DISKUS inhalation powder 100/50 mcg is supplied as a disposable purple plastic inhaler containing a foil blister strip with 60 blisters. The inhaler is packaged in a plastic-coated, moisture-protective foil pouch (NDC 66993-584-97). Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg is supplied as a disposable purple plastic inhaler containing a foil blister strip with 60 blisters. The inhaler is packaged in a plastic-coated, moisture-protective foil pouch (NDC 66993-585-97). Fluticasone Propionate/Salmeterol DISKUS inhalation powder 500/50 mcg is supplied as a disposable purple plastic inhaler containing a foil blister strip with 60 blisters. The inhaler is packaged in a plastic-coated, moisture-protective foil pouch (NDC 66993-586-97). Store at room temperature between 68°F and 77°F (20°C and 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Store in a dry place away from direct heat or sunlight. Keep out of reach of children. Fluticasone Propionate/Salmeterol DISKUS should be stored inside the unopened moisture-protective foil pouch and only removed from the pouch immediately before initial use. Discard Fluticasone Propionate/Salmeterol DISKUS 1 month after opening the foil pouch or when the counter reads “0” (after all blisters have been used), whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
Indications & Usage
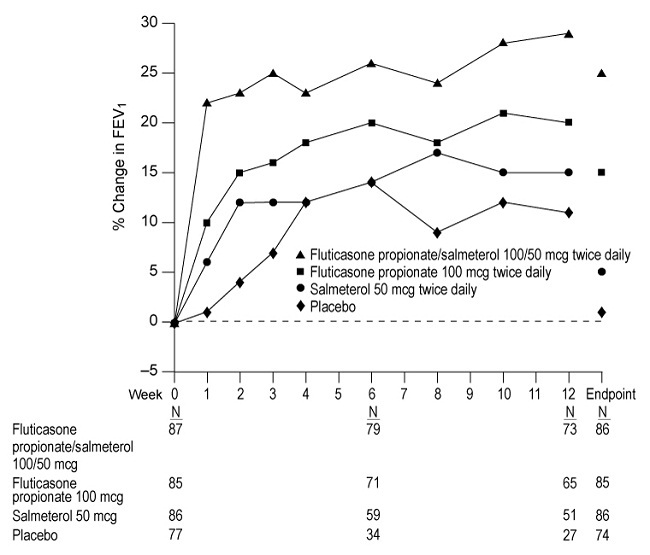
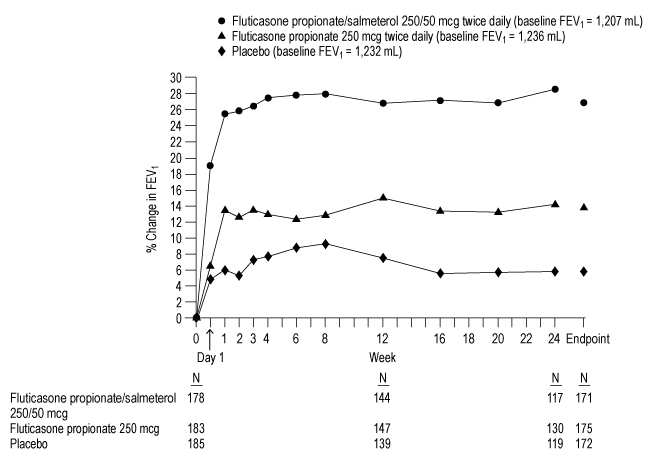
1 INDICATIONS AND USAGE Fluticasone Propionate/Salmeterol DISKUS is a combination product containing a corticosteroid and a long-acting beta 2 -adrenergic agonist (LABA) indicated for: • Twice-daily treatment of asthma in patients aged 4 years and older. ( 1.1 ) • Maintenance treatment of airflow obstruction and reducing exacerbations in patients with chronic obstructive pulmonary disease (COPD). ( 1.2 ) Important limitation of use: Not indicated for relief of acute bronchospasm. ( 1.1 , 1.2 ) 1.1 Treatment of Asthma Fluticasone Propionate/Salmeterol DISKUS is indicated for the twice-daily treatment of asthma in patients aged 4 years and older. Fluticasone Propionate/Salmeterol DISKUS should be used for patients not adequately controlled on a long-term asthma control medication such as an inhaled corticosteroid (ICS) or whose disease warrants initiation of treatment with both an ICS and long-acting beta 2 -adrenergic agonist (LABA). Important Limitation of Use Fluticasone Propionate/Salmeterol DISKUS is NOT indicated for the relief of acute bronchospasm. 1.2 Maintenance Treatment of Chronic Obstructive Pulmonary Disease Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg is indicated for the twice-daily maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema. Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg is also indicated to reduce exacerbations of COPD in patients with a history of exacerbations. Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg twice daily is the only approved dosage for the treatment of COPD because an efficacy advantage of the higher strength Fluticasone Propionate/Salmeterol DISKUS inhalation powder 500/50 mcg over Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg has not been demonstrated. Important Limitation of Use Fluticasone Propionate/Salmeterol DISKUS is NOT indicated for the relief of acute bronchospasm.
Dosage and Administration
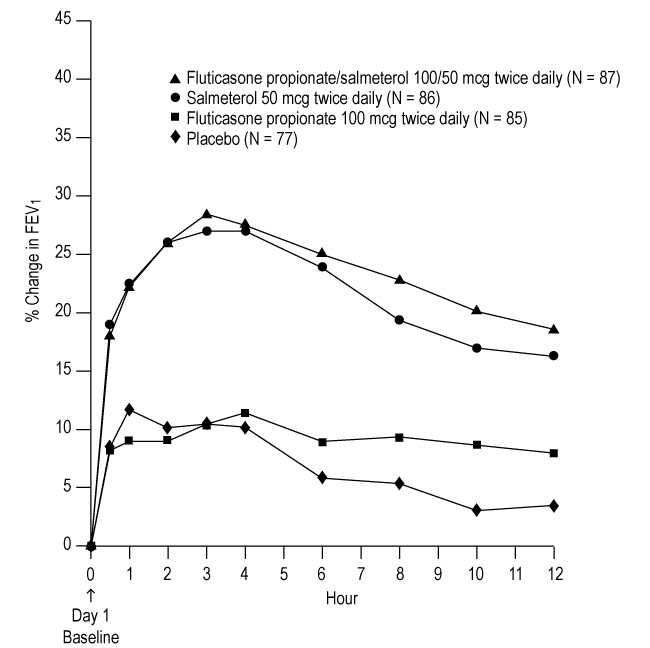
2 DOSAGE AND ADMINISTRATION Fluticasone Propionate/Salmeterol DISKUS should be administered as 1 inhalation twice daily by the orally inhaled route only. After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis. More frequent administration or a greater number of inhalations (more than 1 inhalation twice daily) of the prescribed strength of Fluticasone Propionate/Salmeterol DISKUS is not recommended as some patients are more likely to experience adverse effects with higher doses of salmeterol. Patients using Fluticasone Propionate/Salmeterol DISKUS should not use additional LABA for any reason. [See Warnings and Precautions ( 5.3 , 5.12 ).] • For oral inhalation only. ( 2 ) • Treatment of asthma in patients aged 12 years and older: 1 inhalation of Fluticasone Propionate/Salmeterol DISKUS inhalation powder 100/50 mcg, Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg, and Fluticasone Propionate/Salmeterol DISKUS inhalation powder 500/50 mcg twice daily. Starting dosage is based on asthma severity. ( 2.1 ) • Treatment of asthma in patients aged 4 to 11 years: 1 inhalation of Fluticasone Propionate/Salmeterol DISKUS inhalation powder 100/50 mcg twice daily. ( 2.1 ) • Maintenance treatment of COPD: 1 inhalation of Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg twice daily. ( 2.2 ) 2.1 Asthma If asthma symptoms arise in the period between doses, an inhaled, short-acting beta 2 -agonist should be taken for immediate relief. Adult and Adolescent Patients Aged 12 Years and Older For patients aged 12 years and older, the dosage is 1 inhalation twice daily, approximately 12 hours apart. When choosing the starting dosage strength of Fluticasone Propionate/Salmeterol DISKUS, consider the patients’ disease severity, based on their previous asthma therapy, including the ICS dosage, as well as the patients’ current control of asthma symptoms and risk of future exacerbation. The maximum recommended dosage is Fluticasone Propionate/Salmeterol DISKUS inhalation powder 500/50 mcg twice daily. Improvement in asthma control following inhaled administration of Fluticasone Propionate/Salmeterol DISKUS can occur within 30 minutes of beginning treatment, although maximum benefit may not be achieved for 1 week or longer after starting treatment. Individual patients will experience a variable time to onset and degree of symptom relief. For patients who do not respond adequately to the starting dosage after 2 weeks of therapy, replacing the current strength of Fluticasone Propionate/Salmeterol DISKUS with a higher strength may provide additional improvement in asthma control. If a previously effective dosage regimen fails to provide adequate improvement in asthma control, the therapeutic regimen should be reevaluated and additional therapeutic options (e.g., replacing the current strength of Fluticasone Propionate/Salmeterol DISKUS with a higher strength, adding additional ICS, initiating oral corticosteroids) should be considered. Pediatric Patients Aged 4 to 11 Years For patients with asthma aged 4 to 11 years who are not controlled on an ICS, the dosage is 1 inhalation of Fluticasone Propionate/Salmeterol DISKUS inhalation powder 100/50 mcg twice daily, approximately 12 hours apart. 2.2 Chronic Obstructive Pulmonary Disease The recommended dosage for patients with COPD is 1 inhalation of Fluticasone Propionate/Salmeterol DISKUS inhalation powder 250/50 mcg twice daily, approximately 12 hours apart. If shortness of breath occurs in the period between doses, an inhaled, short-acting beta 2 -agonist should be taken for immediate relief.
