Drug Catalog - Product Detail
FLUTICASONE PROPIONATE CREAM CRM 0.0005 15GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0222-35 | PADAGIS | 15 | 0.05% | CREAM |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
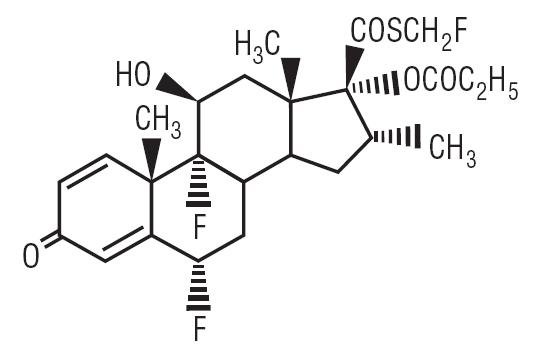
Description Fluticasone Propionate Cream, 0.05% contains fluticasone propionate [(6α,11β,16α,17α)-6,9,-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid, S-fluoromethyl ester], a synthetic fluorinated corticosteroid, for topical dermatologic use. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. Chemically, fluticasone propionate is C25H31F3O5S. It has the following structural formula: Fluticasone propionate has a molecular weight of 500.6. It is a white to off-white powder and is insoluble in water. Each gram of Fluticasone Propionate Cream, 0.05% contains fluticasone propionate 0.5 mg in a base of ceteth-20, cetostearyl alcohol, citric acid, dibasic sodium phosphate, isopropyl myristate, mineral oil, propylene glycol, and purified water, with imidurea as a preservative. Structural formula
How Supplied
How supplied Fluticasone Propionate Cream, 0.05% is available as follows: 15 g tube (NDC 45802-222-35) 30 g tube (NDC 45802-222-11) 60 g tube (NDC 45802-222-37)
Indications & Usage
INdications & Usage Fluticasone Propionate Cream, 0.05% is a medium potency corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Fluticasone Propionate Cream, 0.05% may be used with caution in pediatric patients 3 months of age or older. The safety and efficacy of drug use for longer than 4 weeks in this population have not been established. The safety and efficacy of Fluticasone Propionate Cream, 0.05% in pediatric patients below 3 months of age have not been established.
Dosage and Administration
Dosage & Administration Fluticasone Propionate Cream, 0.05% may be used in adult and pediatric patients 3 months of age or older. Safety and efficacy of Fluticasone Propionate Cream, 0.05% in pediatric patients for more than 4 weeks of use have not been established (see PRECAUTIONS - PEDIATRIC USE). The safety and efficacy of Fluticasone Propionate Cream, 0.05% in pediatric patients below 3 months of age have not been established. Atopic Dermatitis - Apply a thin film of Fluticasone Propionate Cream, 0.05% to the affected skin areas once or twice daily. Rub in gently. Other Corticosteroid-Responsive Dermatoses - Apply a thin film of Fluticasone Propionate Cream, 0.05% to the affected skin areas twice daily. Rub in gently. As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. Fluticasone Propionate Cream, 0.05% should not be used with occlusive dressings. Fluticasone Propionate Cream, 0.05% should not be applied in the diaper area, as diapers or plastic pants may constitute occlusive dressings. Geriatric Use - In studies where geriatric patients (65 years of age or older, see PRECAUTIONS) have been treated with fluticasone propionate cream, 0.05%, safety did not differ from that in younger patients; therefore, no dosage adjustment is recommended.
