Drug Catalog - Product Detail
FLURBIPROFEN SODIUM SOL 0.0003 2.5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24208-0314-25 | BAUSCH HEALTH | 2 | 0.03% | SOLUTION |
PACKAGE FILES

Generic Name
FLURBIPROFEN SODIUM
Substance Name
FLURBIPROFEN SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
OPHTHALMIC
Application Number
ANDA074447
Description
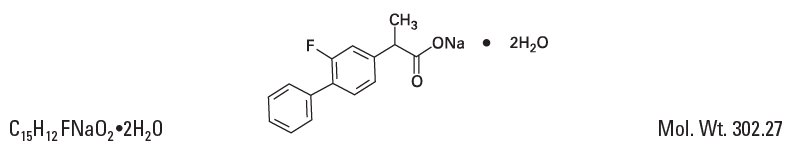
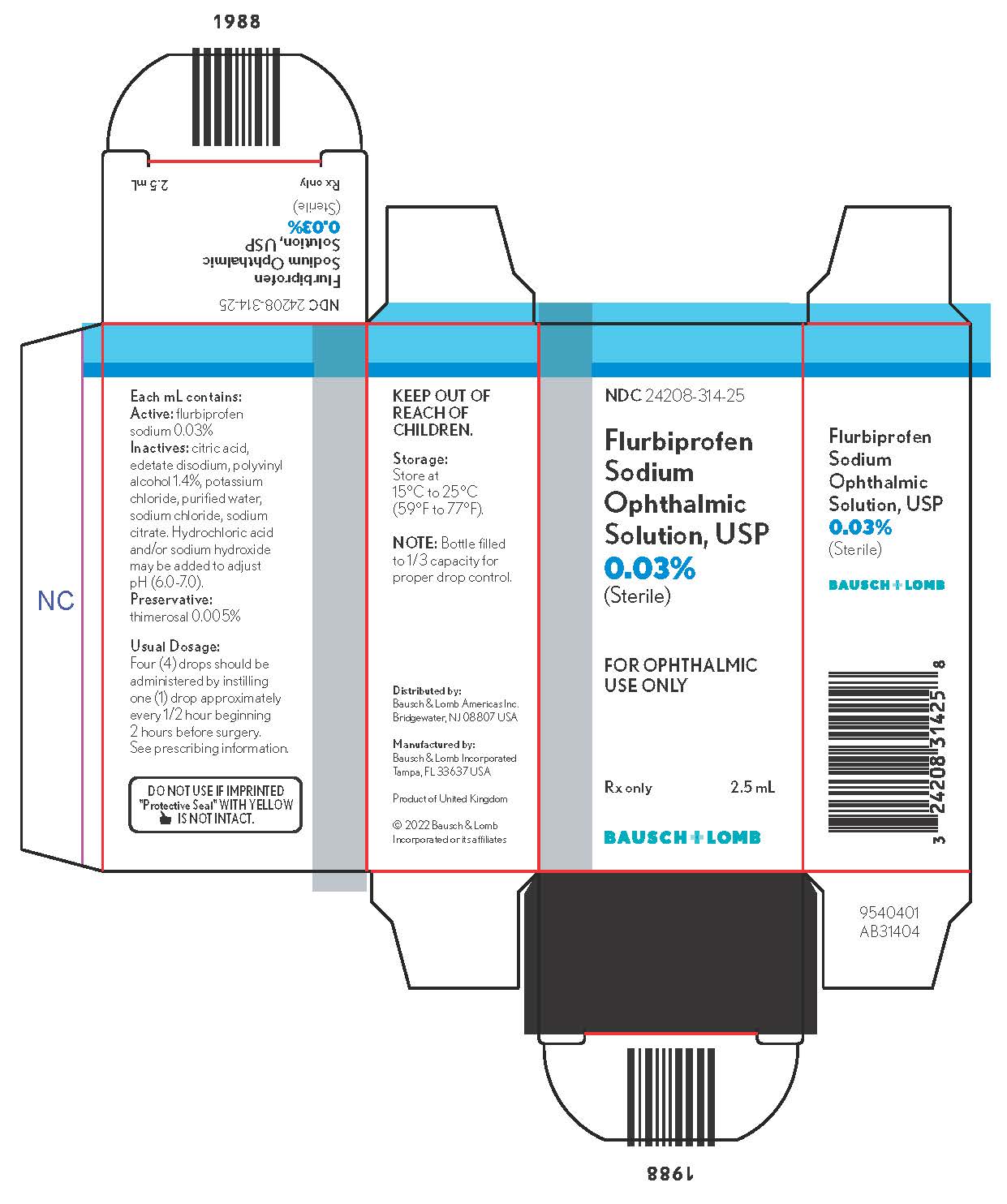
DESCRIPTION Flurbiprofen sodium ophthalmic solution USP, 0.03% is a sterile topical nonsteroidal anti-inflammatory product for ophthalmic use. Chemical Name: Sodium (±)-2-(2-fluoro-4-biphenylyl)propionate dihydrate. Structural Formula: Each mL contains: Active: flurbiprofen sodium 0.03%. Inactives: citric acid, edetate disodium, polyvinyl alcohol 1.4%, potassium chloride, purified water, sodium chloride, sodium citrate. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (6.0 – 7.0). Preservative: thimerosal 0.005%. Diagram Description automatically generated
How Supplied
HOW SUPPLIED Flurbiprofen sodium ophthalmic solution USP, 0.03% is supplied in a plastic bottle with a controlled drop tip in the following size: 2.5 mL - NDC 24208-314-25 DO NOT USE
Indications & Usage
INDICATIONS AND USAGE Flurbiprofen sodium ophthalmic solution is indicated for the inhibition of intraoperative miosis.
Dosage and Administration
DOSAGE AND ADMINISTRATION A total of four (4) drops of flurbiprofen sodium ophthalmic solution should be administered by instilling one (1) drop approximately every 1/2 hour beginning 2 hours before surgery.
