Drug Catalog - Product Detail
FLUOXETINE HCL CAPS. CP 90MG 4
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 55111-0284-48 | DR.REDDY'S LABORATORIES, INC. | 4 | 90MG | CAPSULE |
PACKAGE FILES

Generic Name
FLUOXETINE HYDROCHLORIDE
Substance Name
FLUOXETINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078572
Description
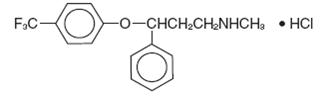
11 DESCRIPTION Fluoxetine delayed-release capsules USP is a selective serotonin reuptake inhibitor for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem ® , fluoxetine hydrochloride). It is designated (±)-N-methyl-3-phenyl-3-[(α,α,α-trifluoro-p-tolyl)oxy]propylamine hydrochloride and has the molecular formula of C 17 H 18 F 3 NO•HCl. Its molecular weight is 345.79. The structural formula is: Fluoxetine hydrochloride USP is a white to off-white crystalline powder. Sparingly soluble in purified water and in methylene chloride; freely soluble in methanol and in alcohol. Practically insoluble in ether. Each delayed release capsule, for oral administration contains white to off-white elliptical to spherical enteric-coated pellets of fluoxetine hydrochloride equivalent to 90 mg (291 µmol) of fluoxetine. The capsules also contain black iron oxide, copovidone, gelatin, glycine, hypromellose, hypromellose phthalate, isopropyl alcohol, methylene chloride, red iron oxide, sugar globules, talc, titanium dioxide, triethyl citrate and yellow iron oxide.
How Supplied
Indications & Usage
1 INDICATIONS AND USAGE Fluoxetine delayed-release capsules are indicated for the treatment of: • Acute and maintenance treatment of Major Depressive Disorder [see Clinical Studies ( 14.1 )] . Fluoxetine delayed-release capsules are selective serotonin reuptake inhibitor indicated for: Acute and maintenance treatment of Major Depressive Disorder (MDD) (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Indication Adult Pediatric MDD (2.1) 20 mg/day in am (initial dose) 10 to 20 mg/day (initial dose) A lower or less frequent dosage should be used in patients with hepatic impairment, the elderly, and for patients with concurrent disease or on multiple concomitant medications ( 2.7) Dosing with fluoxetine weekly capsules - initiate 7 days after the last daily dose of fluoxetine 20 mg ( 2.1 ) 2.1 Major Depressive Disorder Initial Treatment Adult — Initiate fluoxetine delayed-release capsules 20 mg/day orally in the morning. Consider a dose increase after several weeks if insufficient clinical improvement is observed. Administer doses above 20 mg/day once daily in the morning or twice daily (i.e., morning and noon).The maximum fluoxetine dose should not exceed 80 mg/day. In controlled trials used to support the efficacy of fluoxetine, patients were administered morning doses ranging from 20 to 80 mg/day. Studies comparing fluoxetine 20, 40, and 60 mg/day to placebo indicate that 20 mg/day is sufficient to obtain a satisfactory response in Major Depressive Disorder in most cases [see Clinical Studies ( 14.1 ) ]. Pediatric (children and adolescents) — Initiate fluoxetine delayed-release capsules, 10 or 20 mg/day. After 1 week at 10 mg/day, increase the dose to 20 mg/day. However, due to higher plasma levels in lower weight children, the starting and target dose in this group may be 10 mg/day. Consider a dose increase to 20 mg/day after several weeks if insufficient clinical improvement is observed. In the short-term (8 to 9 week) controlled clinical trials of fluoxetine supporting its effectiveness in the treatment of Major Depressive Disorder, patients were administered fluoxetine doses of 10 to 20 mg/day [see Clinical Studies ( 14.1 ) ]. All patients — As with other drugs effective in the treatment of Major Depressive Disorder, the full effect may be delayed until 4 weeks of treatment or longer. Periodically reassess to determine the need for maintenance treatment. Weekly Dosing — Initiate fluoxetine delayed-release capsules (once-weekly) 7 days after the last daily dose of fluoxetine 20 mg [see Clinical Pharmacology ( 12.3 ) ]. If satisfactory response is not maintained with fluoxetine delayed-release capsules once-weekly, consider reestablishing a daily dosing regimen [see Clinical Studies (14.1) ]. Switching Patients to a Tricyclic Antidepressant (TCA) — Dosage of a TCA may need to be reduced, and plasma TCA concentrations may need to be monitored temporarily when fluoxetine is coadministered or has been recently discontinued [see Warnings and Precautions (5.2) and Drug Interactions (7.7) ]. 2.7 Dosing in Specific Populations Treatment of Pregnant Women — When treating pregnant women with fluoxetine, the physician should carefully consider the potential risks and potential benefits of treatment. Neonates exposed to SSRIs or SNRIs late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding [see Use in Specific Populations (8.1) ]. Geriatric — Consider a lower less frequent dosage for the elderly [see Use in Specific Populations (8.5) ] Hepatic Impairment — As with many other medications, use a lower or less frequent dosage in patients with hepatic impairment [see Clinical Pharmacology (12.4) and Use in Specific Populations (8.6) ]. Concomitant Illness — Patients with concurrent disease or on multiple concomitant medications may require dosage adjustments [see Clinical Pharmacology (12.4) and Warnings and Precautions (5.12 ) ]. 2.8 Discontinuation of Treatment Symptoms associated with discontinuation of fluoxetine, SNRIs, and SSRIs, have been reported [see Warnings and Precautions ( 5.15 )] 2.9 Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with fluoxetine. Conversely, at least 5 weeks should be allowed after stopping fluoxetine before starting an MAOI intended to treat psychiatric disorders [see Contraindications (4.1) ]. 2.10 Use of Fluoxetine with Other MAOIs such as Linezolid or Methylene Blue Do not start fluoxetine in a patient who is being treated with linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered [see Contraindications (4.1) ]. In some cases, a patient already receiving fluoxetine therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, fluoxetine should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for five weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with fluoxetine may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue [see Warnings and Precautions (5.2) ]. The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with fluoxetine is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use [see Warnings and Precautions (5.2) ].
